|
植物來源 |
|
|
生物活性 |
|
|
鑑定 |
熔點 |
178-180°C, 190-191°C |
| 旋光度 |
[α]22D-157 (c, 1水) |
1HNMR

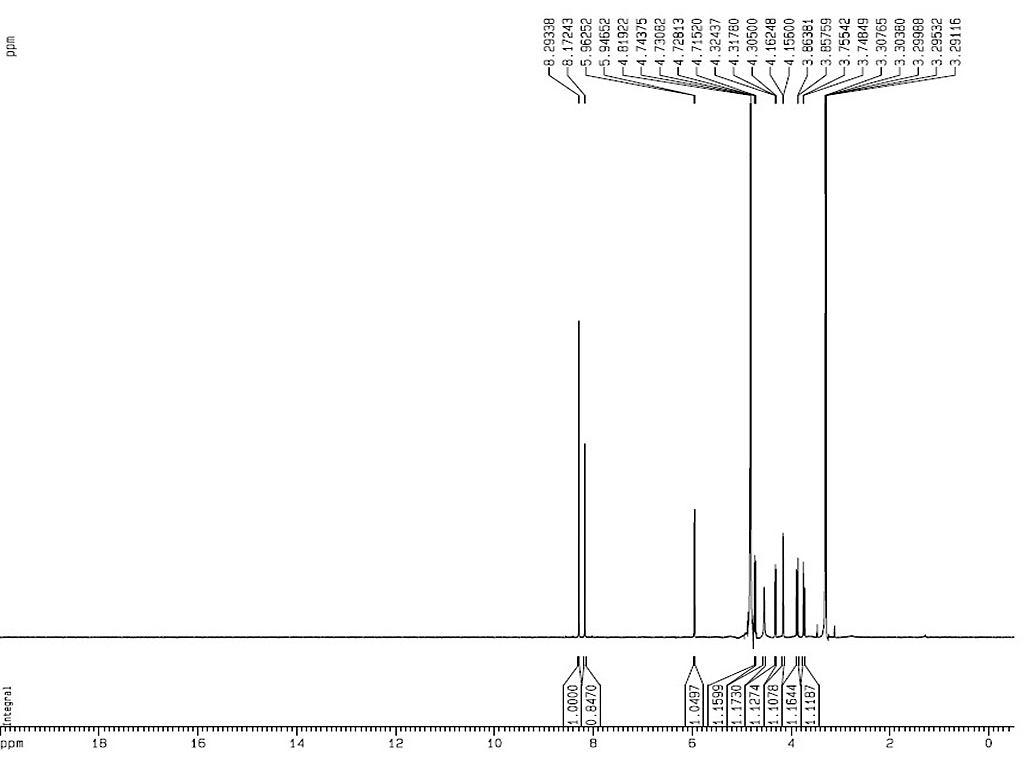
|
| 分析方法 |
|
| 儀器 |
矽膠 GF254 TLC 板 |
| 流動相 |
系統1: 二氯甲烷-甲醇-甲酸 (10: 2: 0.08); 系統2: 二氯甲烷-丙酮-甲酸 (4: 7: 0.12); 系統3: 石油醚-丙酮-甲酸 (3: 8: 0.12) |
| 檢測器 |
UV λ254 nm |
|
|
|
| 儀器 |
Agilent 1100 HPLC儀 |
| 色譜柱 |
Phenomenex-ODS 色譜柱 (250 mm × 4.6 mm, 5 µm), 30°C |
| 流動相 |
磷酸 pH3: 甲醇 = 90: 10, 1.0 mL/min |
| 檢測器 |
UV λ217 nm |
|
|
|
| 儀器 |
LC系統包括Waters (Milford, MA, USA) model 6000A 泵, Waters model U6K 進樣器, Waters model 680 梯度自動控制器, Waters model 996 光電二極體檢測器, Waters Empower 2 資料收集軟體及Primesphere NH2 (250 9 4.6 mm; 5) Phenomenex (Torrance, CA, USA) |
| 色譜柱 |
2 cm LC-18 保護柱 (Supelco, Bellefonte, PA, USA) |
| 流動相 |
A: pH 4.8 KH2PO4, B: 甲醇, 0-12 min 50% A/ 50% B, 12-20 min 50-100% B. 20-30 min 100% 甲醇, 30-45 min 平衡; 進樣量: 10 µL, 1.0 mL/min |
| 檢測器 |
UV λ210 nm |
|
|
|
| 儀器 |
Agilent LC 1100 系統包括一個二極管數組檢測器和質譜檢測器 (Agilent Technologies, Palo Alto, CA, USA); LC 系統包括一個四元泵 (Model G1311A), 一個自動進樣器 (Model G1313A), 一個脫氣機 (Model G1379A), 光電二極管數組檢測器 (Model G1315B) |
| 色譜柱 |
Synergi-Max 反相色譜柱 (150 × 4.6 mm I.D.; 4 µm (Phenomenex)) 及一個保護柱 (Supelco) |
| 流動相 |
A: 0.1% 乙酸, B: 0.1% 乙酸乙腈, 0-3 min 95% A/ 5% B 保持, 3-10 min 5-100% B, 10-15 min 100 % 乙腈, 15-30 min 95% A/ 5% B; 0.5 mL/min |
| 檢測器 |
飛行時間檢測器 (Model G1969A) 包括一個電噴霧離子源和 Agilent 軟件 (Agilent MassHunter 工作站, A.02.01), 負離子模式, 毛細管電壓: 3000 V, 霧化氣和乾燥氣: 氮氣13 L/min 350°C, PMT 電壓: 800 V, 碎片電壓: 100 V, 撇渣器電壓: 60 V, 全掃描 m/z 100-400 |
|
| 樣品製備 |
|
方法一 |
|
|
95% 乙醇室溫提取樣品 24 小時. 過濾, 重複四次。合幷, 40°C 真空濃縮. |
|
|
利用甲苯和水分餾乙醇提取物. 減壓濃縮甲苯餾分. 乙酸乙酯分餾水部, 40°C 減壓濃縮. 正丁醇分餾水部, 濃縮. |
|
|
正丁醇餾分過矽膠色譜柱 (60-120 mesh), 己烷和乙酸乙酯洗脫. 收集, 利用 TLC 分析 (流動相: 正丁醇: 乙酸乙酯: 水 (4: 1: 5); UV λ254 nm), 6% 甲醇的乙酸乙酯中. 重複以純化. |
|
|
方法二 |
|
|
Soxhlet 提取器用 95% 乙醇提取樣品粉末 2 小時. 蒸幹, 80°C 水溶解. 回流 5 min 加四-五滴 37-40% 甲醛. 過濾, 降溫 |
|
|
過負離子交換柱 (Amberlite IRA-400, 乙酸, 淨重25 g). 25% 乙酸溶液洗脫. 收集, 濃縮。甲醇溶解, 加熱 10 min 活化. 過濾收集沉澱物, 蒸幹. |
|
|
甲醇和甲苯 (或乙酸乙酯) 重結晶得到目標化合物. |
|
|
方法三 |
|
|
風乾樣品 24 小時再 65°C 烘乾. 去離子水浸提 4 小時. 重複三次. |
|
|
提取物過 Amberlite IRA-400 柱 (乙酸), 25% 乙酸洗脫. 濃縮, 乙酸乙酯和甲醇重結晶得目標化合物. |
|
|
| 參考文獻 |
|
[1]
|
Orhan, N., et al. (2012). "Identification of hypoglycaemic compounds from berries of Juniperus oxycedrus subsp. oxycedrus through bioactivity guided isolation technique." Journal of Ethnopharmacology 139(1): 110-118. |
|
[2]
|
Wang, G.-W., et al. (2011). "Illicium verum: A review on its botany, traditional use, chemistry and pharmacology." Journal of Ethnopharmacology 136(1): 10-20. |
|
[3]
|
Guimarães, R., et al. (2013). "Infusion and decoction of wild German chamomile: Bioactivity and characterization of organic acids and phenolic compounds." Food Chemistry 136(2): 947-954. |
|
[4]
|
Liu, D., et al. (2011). "Separation of shikimic acid from pine needles of Cedrus deodara and its purity determination." Zhongguo Xiandai Yingyong Yaoxue 28(7): 637-640. |
|
[5]
|
Martin, E., et al. (2010). "Sweetgum (Liquidambar styraciflua L.): Extraction of Shikimic Acid Coupled to Dilute Acid Pretreatment." Applied Biochemistry and Biotechnology 162(6): 1660-1668. |
|
[6]
|
Matallo, M. B., et al. (2009). "EXTRACTION and analysis of shikimic acid from plant tissues." Planta Daninha 27: 987-994. |
|
[7]
|
Rawat, G., et al. (2013). "A Natural Isolate Producing Shikimic Acid: Isolation, Identification, and Culture Condition Optimization." Applied Biochemistry and Biotechnology 169(8): 2290-2302. |
|
[8]
|
Prasad, J., et al. (2012). "Antidyslipidemic and antioxidant activity of the constituents isolated from the leaves of Calophyllum inophyllum." Phytomedicine 19(14): 1245-1249. |
|
[9]
|
Orhan, N., et al. (2012). "A bioactivity guided study on the antidiabetic activity of Juniperus oxycedrus subsp. oxycedrus L. leaves." Journal of Ethnopharmacology 140(2): 409-415. |
|
[10]
|
Oliver, C. E., et al. (2010). "Effect of chlorate, molybdate, and shikimic acid on Salmonella enterica serovar Typhimurium in aerobic and anaerobic cultures." Anaerobe 16(2): 106-113. |
|
[11]
|
Morucci, F., et al. (2012). "Antinociceptive activity of aqueous extract and isolated compounds of Lithrea molleoides." J Ethnopharmacol 142(2): 401-406. |
|
[12]
|
Papetti, A., et al. (2013). "Identification of organic acids in Cichorium intybus inhibiting virulence-related properties of oral pathogenic bacteria." Food Chemistry 138(2–3): 1706-1712. |
|
[13]
|
Colombo, S. L., et al. (1998). "The interaction of shikimic acid and protein phosphorylation with pep carboxylase from the C4 dicot Amaranthus viridis." Phytochemistry 48(1): 55-59. |
|
[14]
|
Liu, D., et al. (2011). "Separation of shikimic acid from pine needles of Cedrus deodara and its purity determination." Zhongguo Xiandai Yingyong Yaoxue 28(7): 637-640. |
|
[15]
|
Avula, B., et al. (2009). "Determination of Shikimic Acid in Fruits of Illicium Species and Various Other Plant Samples by LC–UV and LC–ESI–MS." Chromatographia 69(3-4): 307-314. |
|
| 連結 |
 中藥材圖像數據庫 中藥材圖像數據庫
 藥用植物圖像數據庫 藥用植物圖像數據庫
 中藥標本數據庫 中藥標本數據庫
|
 中藥材圖像數據庫
中藥材圖像數據庫
 藥用植物圖像數據庫
藥用植物圖像數據庫
 中藥標本數據庫
中藥標本數據庫


