|
Natural Resources |
|
|
Bioactivities |
|
|
Identification |
Melting point |
178-180°C, 190-191°C |
| Optical rotation |
[α]22D-157 (c, 1 in H2O) |
1HNMR

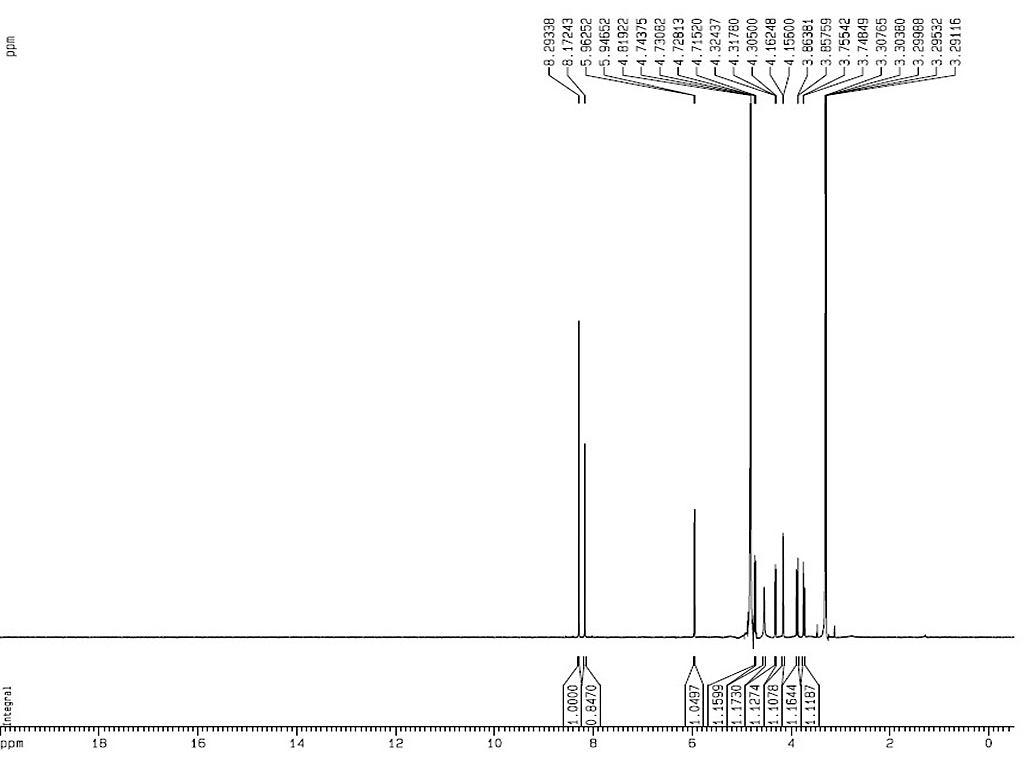
|
| Analytical Method |
|
| INSTRUMENT |
Silica gel GF254 TLC plate |
| MOBILE PHASE |
System 1: methylene chloride-methanol-formic acid (10: 2: 0.08); system 2: methylene chloride-acetone-formic acid (4: 7: 0.12); system 3: petroleum ether-acetone-formic acid (3: 8: 0.12) |
| DETECTION |
UV λ254 nm |
|
|
|
| INSTRUMENT |
Agilent 1100 HPLC |
| COLUMN |
Phenomenex-ODS (250 mm × 4.6 mm, 5 µm), 30°C |
| MOBILE PHASE |
H3PO4 pH3: methanol = 90: 10, 1.0 mL/min |
| DETECTION |
UV λ217 nm |
|
|
|
| INSTRUMENT |
LC system consisted of Waters (Milford, MA, USA) model 6000A pumps, a Waters model U6K injector, a Waters model 680 automated gradient controller, a Waters model 996 photodiode array detector, and a computerized data station equipped with Waters Empower 2 software on a Primesphere NH2 (250 9 4.6 mm; 5 particle size) from Phenomenex (Torrance, CA, USA) |
| COLUMN |
2 cm LC-18 guard column (Supelco, Bellefonte, PA, USA) |
| MOBILE PHASE |
A: pH 4.8 KH2PO4, B: MeOH, 0-12 min 50% A/ 50% B, 12-20 min 50-100% B. 20-30 min 100% methanol, 30-45 min equilibration; injection volume: 10 µL, 1.0 mL/min |
| DETECTION |
UV λ210 nm |
|
|
|
| INSTRUMENT |
Agilent LC 1100 series equipped with a diode array detector and mass detector in series (Agilent Technologies, Palo Alto, CA, USA); LC system with a quaternary pump (Model G1311A), an autosampler (Model G1313A), a degasser (Model G1379A), and a photodiode array detector (Model G1315B) |
| COLUMN |
Synergi-Max RP column (150 × 4.6 mm I.D.; 4 µm particle size (Phenomenex)) with a guard column (Supelco) |
| MOBILE PHASE |
A: 0.1% acetic acid, B: acetonitrile with 0.1% acetic acid, 0-3 min 95% A/ 5% B held, 3-10 min 5-100% B, 10-15 min 100 % acetonitrile, 15-30 min 95% A/ 5% B; 0.5 mL/min |
| DETECTION |
Time-of-flight (Model G1969A) with an electrospray ionization interface and Agilent software (Agilent MassHunter Work Station, A.02.01), negative ionization mode, capillary voltage: 3000 V, nebulizer gas and drying gas: nitrogen 13 L/min 350°C, PMT voltage: 800 V, fragmentor voltage: 100 V, skimmer voltage: 60 V, Full scan m/z 100-400 |
|
| Sample Preparation |
|
METHOD 1 |
|
|
Place powdered leaves of Calophyllum inophyllum in glass percolator with 95% ethanol and allow it to stand at room temperature for 24 hours. Filter and collect the filtrate. Repeat the above four times. Combine and concentrate the filtrates under vacuum using rotavapor at 40°C. |
|
|
Partition the ethanol extract between toluene and water. Separate the two fractions with separating funnel. Concentrate the toluene soluble fraction under reduced pressure. Partition the aqueous fraction with ethyl acetate in separating funnel and concentrate ethyl acetate soluble fraction under reduced pressure by rotavapor at 40°C. Fractionate the aqueous fraction again with n-butanol. Concentrate the water soluble fraction. |
|
|
Apply n-butanol fraction to gross column chromatography with silica gel (60-120 mesh size) with hexane and ethyl acetate in increasing polarity. Collect the fraction, which show a single spot on TLC (mobile phase: n-butanol: acetic acid: water (4: 1: 5); UV λ254 nm), in ethyl acetate with 6% methanol. Repeat the above to give pure compound. |
|
|
METHOD 2 |
|
|
Ground and extract Illicium anisatum seeds in a Soxhlet extractor with 95% ethanol for 2 hours. Evaporate the extract to brown viscous oil. Dissolve the brown oil in water and heat that to 80°C. Flux the hot liquor for 5 minutes and add five droplets of a 37-40% formaldehyde solution to it. Filter the mixture to give an orange clear solution after cooling down. |
|
|
Pass the solution through an anion exchange column (Amberlite IRA-400, acetate form, dry weight 25 g). Elute shikimic acid with 25% aqueous acetic acid. Collect and concentrate the yellow eluent. Dissolve the solid residue in methanol and heat it for 10 min using activated carbon. Collect the dingy white solid residue after filtration and evaporation. |
|
|
Recrystallize the solid residue from methanol and toluene (or ethyl acetate)to obtain white crystals. |
|
|
METHOD 3 |
|
|
Air-dry fruit coatings and leaves of the Liquidambar genus trees for 24 hours and oven-dry at 65°C. Soak the milled material in a tenfold volume of deionized water for 4 hours. Repeat the deionized water extraction for three times. |
|
|
Pass the resultant extracts through Amberlite IRA-400 (acetate form), and elute shikimic acid with 25% acetic acid. Concentrate the eluate and crystallize shikimic acid from the mixed ethyl acetate and methanol. |
|
|
| Reference |
|
[1]
|
Orhan, N., et al. (2012). "Identification of hypoglycaemic compounds from berries of Juniperus oxycedrus subsp. oxycedrus through bioactivity guided isolation technique." Journal of Ethnopharmacology 139(1): 110-118. |
|
[2]
|
Wang, G.-W., et al. (2011). "Illicium verum: A review on its botany, traditional use, chemistry and pharmacology." Journal of Ethnopharmacology 136(1): 10-20. |
|
[3]
|
Guimarães, R., et al. (2013). "Infusion and decoction of wild German chamomile: Bioactivity and characterization of organic acids and phenolic compounds." Food Chemistry 136(2): 947-954. |
|
[4]
|
Liu, D., et al. (2011). "Separation of shikimic acid from pine needles of Cedrus deodara and its purity determination." Zhongguo Xiandai Yingyong Yaoxue 28(7): 637-640. |
|
[5]
|
Martin, E., et al. (2010). "Sweetgum (Liquidambar styraciflua L.): Extraction of Shikimic Acid Coupled to Dilute Acid Pretreatment." Applied Biochemistry and Biotechnology 162(6): 1660-1668. |
|
[6]
|
Matallo, M. B., et al. (2009). "EXTRACTION and analysis of shikimic acid from plant tissues." Planta Daninha 27: 987-994. |
|
[7]
|
Rawat, G., et al. (2013). "A Natural Isolate Producing Shikimic Acid: Isolation, Identification, and Culture Condition Optimization." Applied Biochemistry and Biotechnology 169(8): 2290-2302. |
|
[8]
|
Prasad, J., et al. (2012). "Antidyslipidemic and antioxidant activity of the constituents isolated from the leaves of Calophyllum inophyllum." Phytomedicine 19(14): 1245-1249. |
|
[9]
|
Orhan, N., et al. (2012). "A bioactivity guided study on the antidiabetic activity of Juniperus oxycedrus subsp. oxycedrus L. leaves." Journal of Ethnopharmacology 140(2): 409-415. |
|
[10]
|
Oliver, C. E., et al. (2010). "Effect of chlorate, molybdate, and shikimic acid on Salmonella enterica serovar Typhimurium in aerobic and anaerobic cultures." Anaerobe 16(2): 106-113. |
|
[11]
|
Morucci, F., et al. (2012). "Antinociceptive activity of aqueous extract and isolated compounds of Lithrea molleoides." J Ethnopharmacol 142(2): 401-406. |
|
[12]
|
Papetti, A., et al. (2013). "Identification of organic acids in Cichorium intybus inhibiting virulence-related properties of oral pathogenic bacteria." Food Chemistry 138(2–3): 1706-1712. |
|
[13]
|
Colombo, S. L., et al. (1998). "The interaction of shikimic acid and protein phosphorylation with pep carboxylase from the C4 dicot Amaranthus viridis." Phytochemistry 48(1): 55-59. |
|
[14]
|
Liu, D., et al. (2011). "Separation of shikimic acid from pine needles of Cedrus deodara and its purity determination." Zhongguo Xiandai Yingyong Yaoxue 28(7): 637-640. |
|
[15]
|
Avula, B., et al. (2009). "Determination of Shikimic Acid in Fruits of Illicium Species and Various Other Plant Samples by LC–UV and LC–ESI–MS." Chromatographia 69(3-4): 307-314. |
|
| Link to |
 Chinese Medicinal Material Images Database Chinese Medicinal Material Images Database
 Medicinal Plant Images Database Medicinal Plant Images Database
 Chinese Medicine Specimen Database Chinese Medicine Specimen Database
|
 Chinese Medicinal Material Images Database
Chinese Medicinal Material Images Database
 Medicinal Plant Images Database
Medicinal Plant Images Database
 Chinese Medicine Specimen Database
Chinese Medicine Specimen Database


