|
Natural Resources |
|
|
Bioactivities |
|
|
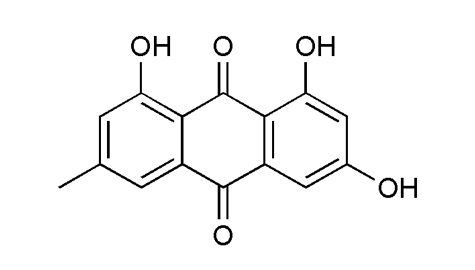
Identification |
Melting point |
253-257°C |
1HNMR

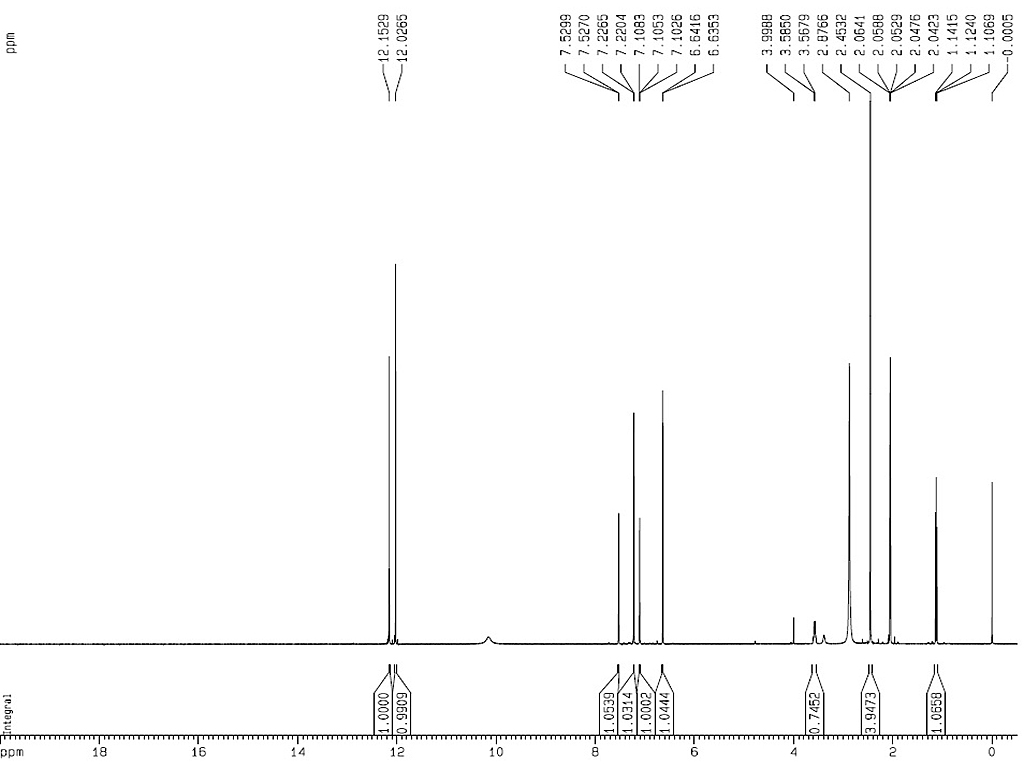
|
| Analytical Method |
|
| INSTRUMENT |
Pre-coated RP-18 F255S HPTLC plates |
| MOBILE PHASE |
Methanol: water: formic acid = 80: 19: 1, v/v/v |
| DETECTION |
UV λ445 nm |
|
|
|
| INSTRUMENT |
Waters model 600E system (Waters, Milford, MA, USA) |
| COLUMN |
Purospher®-Star RP-18e column (4.6 mm i.d. × 250 mm, 5 μm, Merck, Darmstadt, Germany) |
| MOBILE PHASE |
A: Acetonitrile: Methanol = 95: 5, v/v, B: water: acetic acid = 99.9: 0.1, v/v, pH 3.5, 0-15 min: 20% A, 15-25 min: 50% A, 25-30 min: 70% A, 30-40 min: 100% A, 0.8 mL/min. |
| DETECTION |
UV λ290 nm |
|
|
|
| INSTRUMENT |
API 3200 Qtrap triple quadrupole mass spectrometer (Applied Biosystem/MDS SCIEX, Foster City, CA, USA), Waters Acquity™ with diode array detector (DAD) |
| COLUMN |
Acquity UPLC BEH C18 column (50 mm × 2.1 mm i.d., 1.7 mm; Waters, Milford, MA, USA) |
| MOBILE PHASE |
A: 100% aqueous buffer = 2.5 mM NH4Ac, pH 7.4, B: 100% acetonitrile, 0.45 mL/min |
| DETECTION |
UV λ254 nm |
|
| Sample Preparation |
|
METHOD 1 |
|
|
|
|
|
Trisep™ 2100GV CEC system (Unimicro Technologies, Pleasanton, CA, USA), comprised a solvent gradient delivery module, a high-voltage power supply (+30 and −30 kV), a variable wavelength UV-vis detector, a micro fluid manipulation module (including a six-port injector) and a data acquisition module. FS-1 Hi-speed homogenizer (Jintan Fuhua Instrument Corporation, Jiangsu, China) and BF2000 nitrogen evaporator (Beijing Bafang Century Science-Tech Corporation, Beijing, China) |
|
|
The polymerization mixture: 320 μL EDMA, 432 μL IBMA, 48 μL MAA, 2.4 mg AIBN (0.3 wt.% with respect to the monomers), and 1200 μL of porogenic solvent (1-propanol and 1,4-butanediol (70/30 v/v)), sonicated for 15 min to obtain a homogeneous solution. Then, injected into the pretreated capillary. The capillary was sealed at both ends with silicon rubber stoppers and was submerged into a thermostated bath at 60°C for 3-5 hours. The resultant monolithic capillary column was washed with methanol for about 2 hours using an HPLC pump to remove unreacted monomers and porogens. The detection window was created next to the end of the monolithic polymer by removing the coating using heater. The ashes of the organic monolith inside the capillary were flushed out by methanol for about 30 min with the HPLC pump. |
|
|
Driven by electroosmotic flow (EOF), as well as pressurized flow and enters into six-port injection valve. A: ACN, B: phosphate buffer, degassed in an ultrasonic bath and for 15 min before use. All solutions were freshly prepared in water and passed through a 0.22 μm membrane filter before use. |
|
|
UV λ220 nm |
|
|
METHOD 2 |
|
|
Dried and powdered rhizomes of R. emodi (250 g) were extracted with hexane, chloroform and ethyl acetate. |
|
|
Chloroform extract upon column purification with ethyl acetate-hexane = 30: 70, v/v |
|
|
| Reference |
|
[1]
|
Xu, L., et al. (2009). "Emodin augments calcium activated chloride channel in colonic smooth muscle cells by Gi/Go protein." European Journal of Pharmacology 615(1–3): 171-176. |
|
[2]
|
Wu, Z.-X., et al. (2008). "Emodin increases Ca2+ influx through L-type Ca2+ channel in guinea pig gallbladder smooth muscle." European Journal of Pharmacology 595(1–3): 95-99. |
|
[3]
|
Chukwujekwu, J. C., et al. (2006). "Emodin, an antibacterial anthraquinone from the roots of Cassia occidentalis." South African Journal of Botany 72(2): 295-297. |
|
[4]
|
Lü, H., et al. (2007). "Rapid separation and determination of structurally related anthraquinones in Rhubarb by pressurized capillary electrochromatography.\3" Journal of Pharmaceutical and Biomedical Analysis 43(1): 352-357. |
|
[5]
|
Verma, S. C., et al. (2005). "Determination and locational variations in the quantity of hydroxyanthraquinones and their glycosides in rhizomes of Rheum emodi using high-performance liquid chromatography." Journal of Chromatography A 1097(1–2): 59-65. |
|
[6]
|
Singh, N. P., et al. (2005). "High-performance thin layer chromatography method for quantitative determination of four major anthraquinone derivatives in Rheum emodi." Journal of Chromatography A 1077(2): 202-206. |
|
[7]
|
Wang, C., et al. (2007). "Emodin induces apoptosis through caspase 3-dependent pathway in HK-2 cells." Toxicology 231(2–3): 120-128. |
|
[8]
|
Wang, R., et al. (2007). "Emodin suppresses interleukin-1β induced mesangial cells proliferation and extracellular matrix production via inhibiting P38 MAPK." Life Sciences 80(26): 2481-2488. |
|
[9]
|
Hsu, C.-M., et al. (2010). "Emodin inhibits the growth of hepatoma cells: Finding the common anti-cancer pathway using Huh7, Hep3B, and HepG2 cells." Biochemical and Biophysical Research Communications 392(4): 473-478. |
|
[10]
|
Chen, G.-l., et al. (2009). "Protective Effect of Emodin against Lipopolysaccharides-induced Corneal Injury in Rats." Chinese Medical Sciences Journal 24(4): 236-240. |
|
[11]
|
Liu, W., et al. (2011). "Sensitive and robust UPLC–MS/MS method to determine the gender-dependent pharmacokinetics in rats of emodin and its glucuronide." Journal of Pharmaceutical and Biomedical Analysis 54(5): 1157-1162. |
|
| Link to |
 Medicinal Plant Images Database Medicinal Plant Images Database
 Chinese Medicine Specimen Database Chinese Medicine Specimen Database
|
 Medicinal Plant Images Database
Medicinal Plant Images Database
 Chinese Medicine Specimen Database
Chinese Medicine Specimen Database


