|
Natural Resources |
|
|
Bioactivities |
|
|
Identification |
Melting point |
115°C |
| Optical rotation |
[α]17D+6.6 (c, 1 in EtOH) |
13CNMR

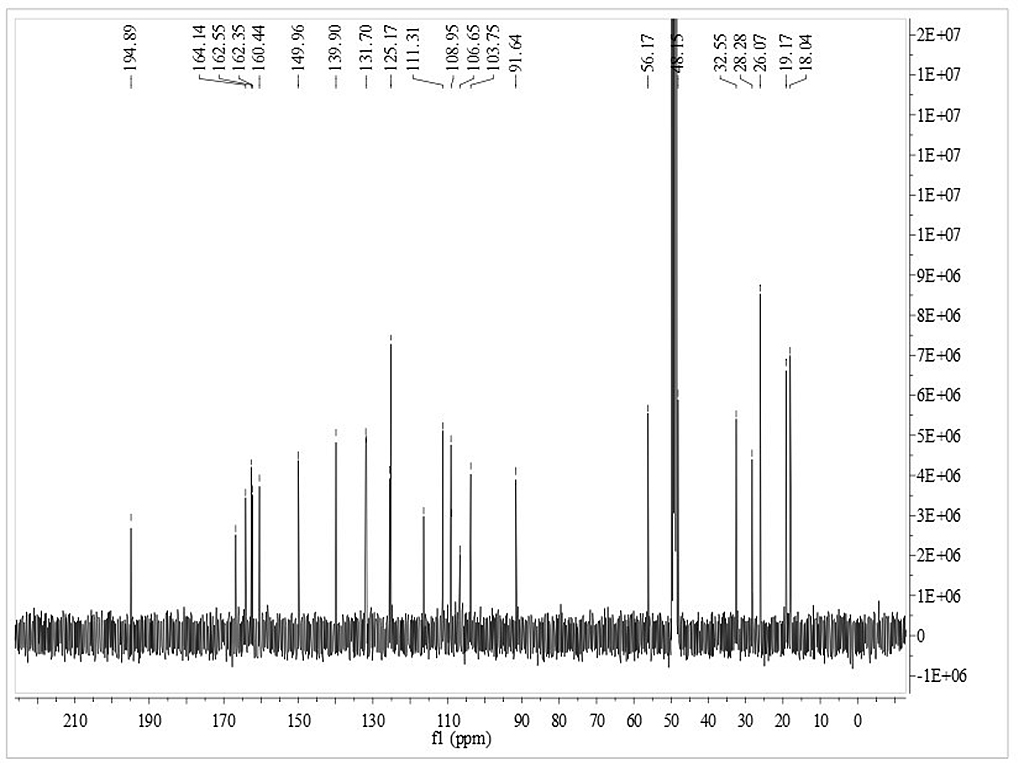
|
| Analytical Method |
|
| INSTRUMENT |
Waters ACQUITY UPLC™ system (Waters Corp., MA, USA) |
| COLUMN |
Waters ACQUITY BEH C18 column (100 × 2.1 mm, 1.7 μm, Waters Corp., Ireland) |
| MOBILE PHASE |
A: acetonitrile, B: water, 0-5 min 40-100% A, 0.3 mL/min |
| DETECTION |
ELSD, drift tube: 75°C, nebulizer: 48°C, gain: 300°C, gas pressure: 45 psi |
|
|
|
| INSTRUMENT |
LC-NMR system (Varian, Palo Alto, CA, USA) |
| COLUMN |
YMC hydrosphere C18 reverse phase column (4.6 x 150 mm, 3µm particle size) |
| MOBILE PHASE |
A; acetonitrile, B: D2O, 0-60 min 45~65% A, 0.8 mL/min |
| DETECTION |
UV λ320 nm, Varian 500 MHz spectrometer equipped with LC-NMR cold probe with a 60µL flow-cell (active volume). Varian WET solvent suppression and scout± scan, free induction decay (FID): 16 K data points, a spectral width of 12000 Hz, a 3µs 90° pulse, a 2 s acquisition time and a 1 s pulse delay. |
|
|
|
| INSTRUMENT |
Waters 1525 HPLC |
| COLUMN |
Phenomenex Luna C18 (250 mm × 4.6 mm, 5µm) column, 35°C |
| MOBILE PHASE |
A: acetonitrile, B: water, 0-35min 45-69% A, 1mL/min |
| DETECTION |
UV λ365 nm |
|
| Sample Preparation |
|
METHOD 1 |
|
|
|
|
|
TBE-1000A high-speed counter-current chromatography (Shanghai, Tauto Biotech, China) which had three polytetrafluoroethylene coils (total volume, 1000 ml) |
|
|
n-hexane: ethyl acetate: methanol: water = 1: 1: 1: 1, v/v, mobile phase: lower phase |
|
|
8.0 mL/min, 500 rpm |
|
|
UV λ254 nm |
|
|
| Reference |
|
[1]
|
Chan, B. C.-L., et al. (2012). "Quick identification of kuraridin, a noncytotoxic anti-MRSA (methicillin-resistant Staphylococcus aureus) agent from Sophora flavescens using high-speed counter-current chromatography." Journal of Chromatography B 880(0): 157-162. |
|
[2]
|
Jung, M. J., et al. (2004). "Isolation of flavonoids and a cerebroside from the stem bark of Albizzia julibrissin." Arch. Pharmacal Res. 27(6): 593-599. |
|
[3]
|
Quang, T. H., et al. (2013). "Anti-Inflammatory and PPAR Transactivational Properties of Flavonoids from the Roots of Sophora flavescens." Phytother. Res. 27(9): 1300-1307. |
|
[4]
|
Sohn, H. Y., et al. (2004). "Antimicrobial and cytotoxic activity of 18 prenylated flavonoids isolated from medicinal plants: Morus alba L., Morus mongolica Schneider, Broussnetia papyrifera (L.) Vent, Sophora flavescens Ait and Echinosophora koreensis Nakai." Phytomedicine 11(7-8): 666-672. |
|
[5]
|
Jeong, T.-S., et al. (2008). "Low density lipoprotein (LDL)-antioxidant flavonoids from roots of Sophora flavescens." Biol Pharm Bull 31(11): 2097-2102. |
|
[6]
|
Han, J.-M., et al. (2010). "Lavandulyl flavonoids from Sophora flavescens suppress lipopolysaccharide-induced activation of nuclear factor-κB and mitogen-activated protein kinases in RAW264.7 cells." Biol. Pharm. Bull. 33(6): 1019-1023. |
|
[7]
|
Chung, M. Y., et al. (2004). "In vitro inhibition of diacylglycerol acyltransferase by prenylflavonoids from Sophora flavescens." Planta Med. 70(3): 258-260. |
|
[8]
|
Kim, S. J., et al. (2003). "Tyrosinase inhibitory prenylated flavonoids from Sophora flavescens." Biol Pharm Bull 26(9): 1348-1350. |
|
[9]
|
Rasul, A., et al. (2011). "Induction of mitochondria-mediated apoptosis in human gastric adenocarcinoma SGC-7901 cells by kuraridin and Nor-kurarinone isolated from Sophora flavescens." Asian Pac J Cancer Prev 12(10): 2499-2504. |
|
[10]
|
Kim, S. J., et al. (2010). "Fast identification of flavonoids in the roots of Sophora flavescens by on-flow LC-NMR." J. Med. Plants Res. 4(23): 2452-2459. |
|
[11]
|
Xu, C., et al. (2008). "HPLC determination of contents of four flavonoids in different samples of Sophora flavescens in market." Zhongyao Xinyao Yu Linchuang Yaoli 19(3): 210-212. |
|
| Link to |
 Chinese Medicinal Material Images Database Chinese Medicinal Material Images Database
 Medicinal Plant Images Database Medicinal Plant Images Database
 Chinese Medicine Specimen Database Chinese Medicine Specimen Database
|
 Chinese Medicinal Material Images Database
Chinese Medicinal Material Images Database
 Medicinal Plant Images Database
Medicinal Plant Images Database
 Chinese Medicine Specimen Database
Chinese Medicine Specimen Database


