|
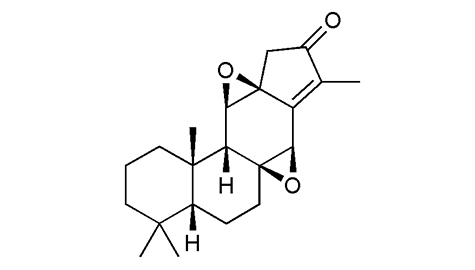
植物來源 |
|
|
生物活性 |
|
|
鑑定 |
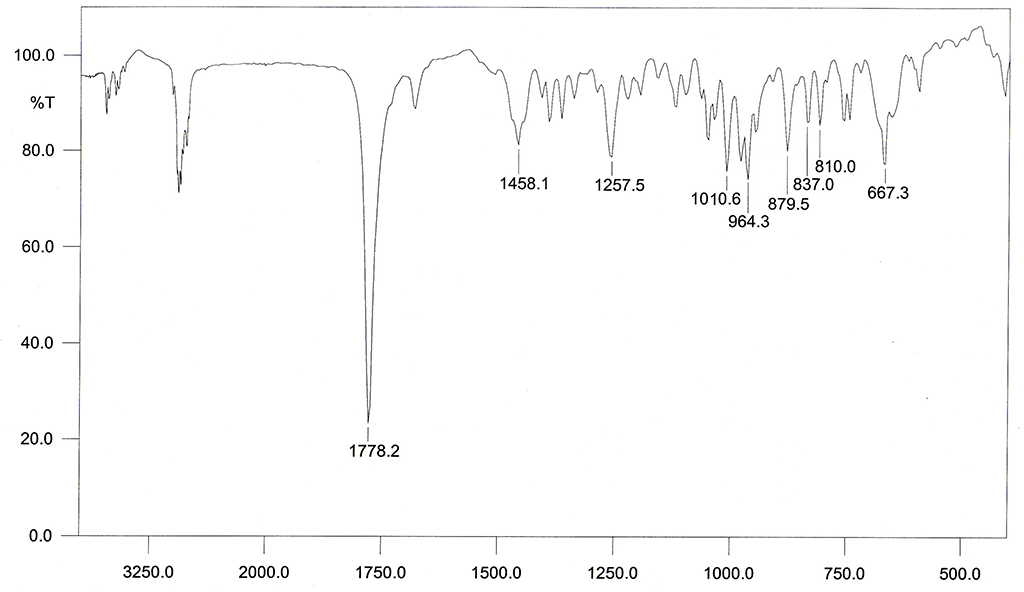
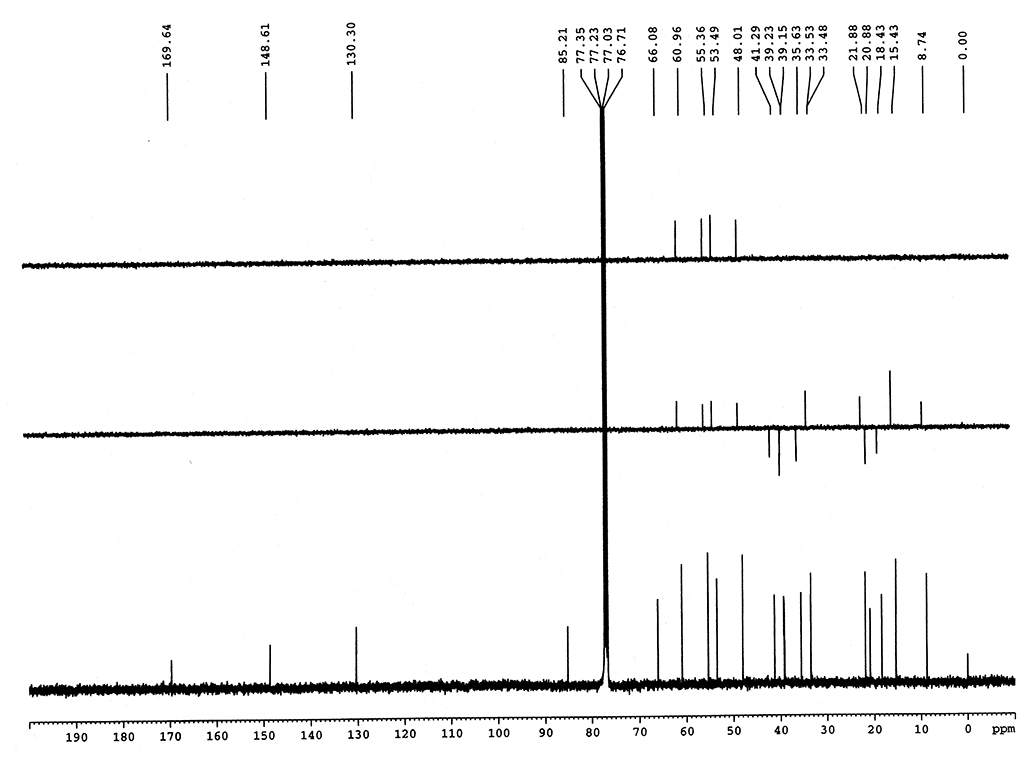
熔點 |
244-244.5°C |
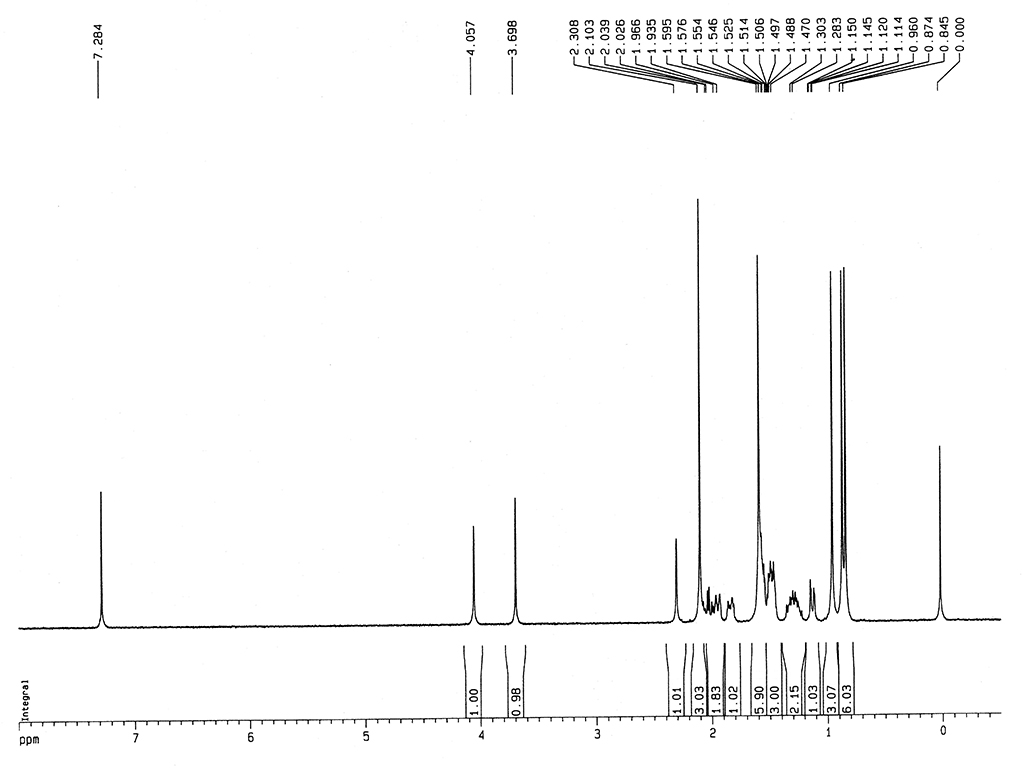
| 旋光度 |
[α]25D+220 (c, 0.4 氯仿) |
|
|
| 分析方法 |
|
| 儀器 |
Agilent-1200 UPLC 系統 (Agilent Corp., USA) |
| 色譜柱 |
Agilent ZORBAX SB-C18 色譜柱 (3.0 mm × 100 mm, I.D., 5 μm) |
| 流動相 |
A: 乙腈ACN, B: 0.1% 甲酸水, 0-20 min 30-65% B, 20-30 min 65-75% B, 30-35 min 75-85% B, 35-60 min 85% B, 60-63 min 等度 30% B. 0.4 mL/min, 20°C |
| 檢測器 |
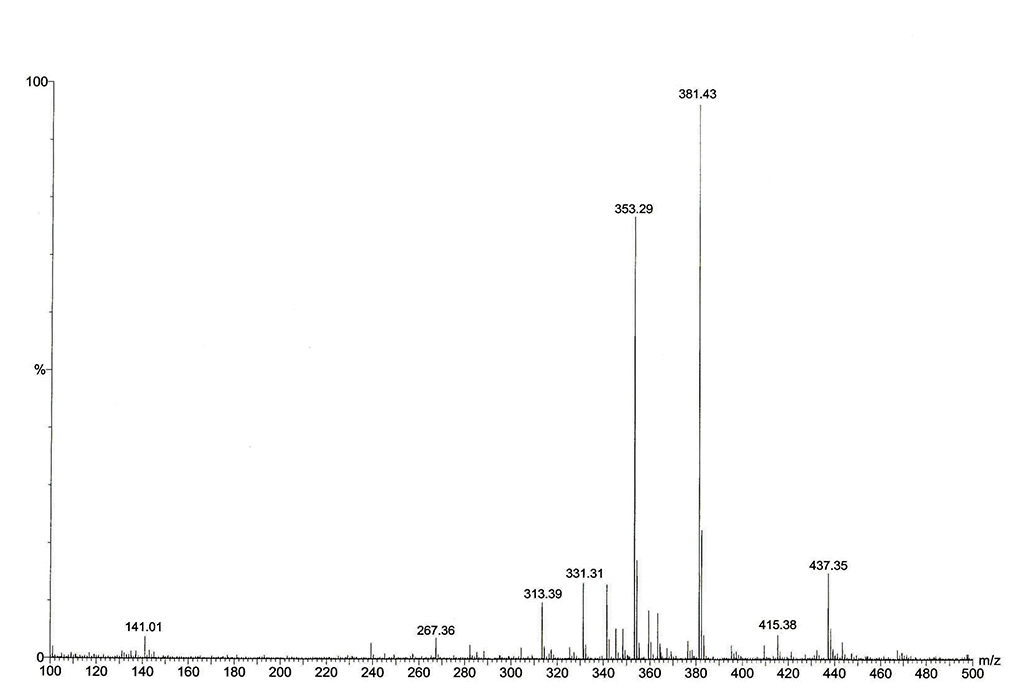
Agilent 6538 UHD Accurate-Mass Q-TOF/MS 質譜儀 (Agilent Corp., USA) 配備ESI 接口, m/z 50-1700 這個鈉離子模式, 10 L/min; 乾燥氣: 350°C ; 霧化氣: 40 psig, 毛細管電壓: 4000 V; 碎片電壓: 175 V; 撇渣器電壓: 65 V; 八極RF: 750 V |
|
|
|
| 儀器 |
Waters 2695 Alliance HPLC 系統 (Waters Corp., Milford, MA, USA) |
| 色譜柱 |
WondaSil™ C18 色譜柱 (GL Science Inc.) 250 mm × 4.6 mm i.d., 5 μm, Waters Symmetry Shield RP C18 保護柱 (20 mm × 3.9 mm, 5 μm) |
| 流動相 |
0-5 min, 60% A; 5-25 min, 60-64% A; 25-26 min, 64-70% A; 26-35 min, 70-75% A; 35-36 min, 75-80% A; 36-50 min, 80-100% A, 1.0 mL/min |
| 檢測器 |
Waters 2424 蒸汽光散射檢測器 (ELSD), 65°C, 氮氣流速 2.7 L/min |
|
|
|
| 儀器 |
LC-10Avp HPLC 儀 (Japan) |
| 色譜柱 |
Lichrospher C18 色譜柱 (4.6 mm × 250 mm, 5 μm) |
| 流動相 |
A: 甲醇, B: 水. 0-5 min, 40% A; 5-10 min, 40-50% A; 10-30 min, 50-55% A; 30-40 min, 55-60% A; 40-45 min, 60-70% A, 1.0 mL/min |
| 檢測器 |
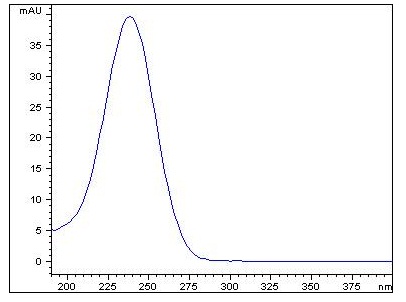
UV λ226 nm |
|
| 參考文獻 |
|
[1]
|
Tang, Y., et al. (2012). "Comparative characteristic of the inflammatory diterpenes in the roots of Euphorbia fischeriana with different preparation method using HPLC-ELSD." Fitoterapia 83(3): 427-433. |
|
[2]
|
Uemura, D. and Y. Hirata (1972). "Two new diterpenoids, jolkinolides A and B, obtained from euphorbia jolkini boiss. (Euphorbiaceae)." Tetrahedron Letters 13(15): 1387-1390. |
|
[3]
|
Wang, C.-J., et al. (2013). "Characterization of phloroglucinol derivatives and diterpenes in Euphorbia ebracteolata Hayata by utilizing ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry." Journal of Pharmaceutical Analysis 3(4): 292-297. |
|
[4]
|
Liu, W. K., et al. (2002). "Jolkinolide B induces neuroendocrine differentiation of human prostate LNCaP cancer cell line." Biochemical Pharmacology 63(5): 951-957. |
|
[5]
|
Huiying, L. and W. Aiqin (2006). "Induction of apoptosis in K562 cells by jolkinolide B." Canadian Journal of Physiology & Pharmacology 84(10): 959-965. |
|
[6]
|
Zhou, S.-s., et al. (2012). "The effects of jolkinolide B on proliferation inhibition of SKOV-3 human ovarian carcinoma cell line." Zhongguo Shenghua Yaowu Zazhi 33(5): 567-570. |
|
[7]
|
Xu, H.-Y., et al. (2013). "Jolkinolide B induces apoptosis in MCF-7 cells through inhibition of the PI3K/Akt/mTOR signaling pathway." Oncol. Rep. 29(1): 212-218. |
|
[8]
|
Lin, Y., et al. (2012). "Jolkinolide B induces apoptosis in MDA-MB-231 cells through inhibition of the PI3K/Akt signaling pathway." Oncol. Rep. 27(6): 1976-1980. |
|
[9]
|
Wang, J.-H., et al. (2011). "Jolkinolide B from Euphorbia fischeriana Steud induces apoptosis in human leukemic U937 cells through PI3K/Akt and XIAP pathways." Mol. Cells 32(5): 451-457. |
|
[10]
|
Lin, Y., et al. (2012). "Effect of jolkinolide B on apoptosis of breast cancer Bcap37 cells." Zhongguo Xinyao Zazhi 21(7): 780-783. |
|
[11]
|
Wang, C., et al. (2011). "RP-HPLC determination of 2,4-dihydroxy-6-methoxy-3-methylacetophenone and jolkinolide B in Euphorbiae Ebracteolatae Radix." Yaowu Fenxi Zazhi 31(5): 839-842. |
|
| 連結 |
 中藥標本數據庫 中藥標本數據庫
|
 中藥標本數據庫
中藥標本數據庫