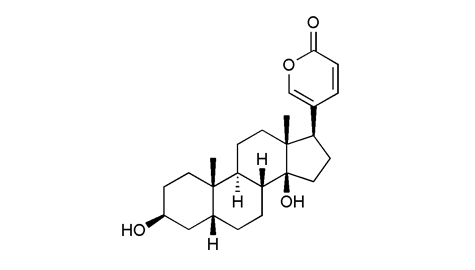
|
植物来源 |
|
|
生物活性 |
|
|
鉴定 |
熔点 |
235-236°C |
| 旋亮度 |
[α]20D-20° |
|
|
| 分析方法 |
|
| 仪器 |
硅胶 60GF 板 (Yantai jiangyou company) |
| 流动相 |
香草素溶液 (2.7 g 香草素, 100 mL 水, 35 mL 浓硫酸), 稀释至300 mL (95% ethanol) |
| 检测器 |
UV 254 nm |
|
|
|
| 仪器 |
硅胶板 |
| 流动相 |
甲醇: 水 (76: 24, v/v) |
|
|
|
| 仪器 |
硅胶GF254 板 (Qingdao Marine Chemical Corporation, China) |
| 流动相 |
石油醚-丙酮 (1: 1, v/v) |
| 检测器 |
10% H2SO4 (乙醇溶液) 喷淋并加热至 120°C 10 min |
|
|
|
| 仪器 |
Agilent 1100 HPLC 仪陪别一个二极管数组检测器 (DAD) 及一个四元液相泵 |
| 色谱柱 |
Zorbax Extend-C18 色谱柱 (4.6 mm × 250 mm, 5 μm) (Agilent, USA) |
| 流动相 |
A: 甲醇, B: 水, 0-6 min 52% A, 6-19 min 52-72% A, 19-29 min 72% A, 1.0 mL/min |
| 检测器 |
UV λ296 nm |
|
|
|
| 仪器 |
Shimazdu LC-20 AD HPLC 仪 |
| 色谱柱 |
C18 Diamonsil® 色谱柱 (50 mm × 2.1 mm, 5 μm) |
| 流动相 |
甲醇-水 (70: 30, 5 mM 乙酸铵, 0.2 mL/min) |
| 检测器 |
API 3000 三重四极质谱, 正离子多反应检测模式, 离子源温度: 400°C, 离子源电压: 4500 V |
|
|
|
| 仪器 |
ACQUITY™ UPLC 仪 (Waters Corp., Milford, MA, USA) 配备一个可调整的自动进样器, 4°C |
| 色谱柱 |
ACQUITY UPLC™ BEH C18 色谱柱 (50 mm × 2.1 mm i.d., 1.7 μm; Waters Corp., Milford, MA, USA), 35°C |
| 流动相 |
A: 乙腈, B: 水 (0.1% 甲酸) |
| 检测器 |
Waters ACQUITY™ TQD 三重四极杆串联质谱 (Waters Corp., Manchester, UK) 通过 ESI 连接 UPLC 仪. 这个钠离子模式, 毛细管电压: 3.8 kV.萃取器及 RF 电压: 2.0 和 0.1 V. 离子源及反溶剂温度: 80 和 400°C. Nitrogen, 反溶剂气: 500 L/h, 锥气: 50 L/h. 碰撞诱导解离 (CID), 碰撞气: 氩气, 0.20 mL/min, 2.81 × 10−3 mbar. 多反应检测 (MRM) |
|
| 参考文献 |
|
[1]
|
Qi, F., et al. (2011). "Antitumor activity of extracts and compounds from the skin of the toad Bufo bufo gargarizans Cantor." International Immunopharmacology 11(3): 342-349. |
|
[2]
|
Gao, H., et al. (2010). "Comparison of toad venoms from different Bufo species by HPLC and LC-DAD-MS/MS." Journal of Ethnopharmacology 131(2): 368-376. |
|
[3]
|
Ferreira, P. M. P., et al. (2013). "Antiproliferative activity of Rhinella marina and Rhaebo guttatus venom extracts from Southern Amazon." Toxicon 72(0): 43-51. |
|
[4]
|
Ma, B., et al. (2013). "Synthesis and structure-activity relationships study of cytotoxic bufalin 3-nitrogen-containing-ester derivatives." Steroids 78(5): 508-512. |
|
[5]
|
Nakano, H., et al. (2005). "Enhancement of ligand-dependent Vitamin D receptor transactivation by the cardiotonic steroid bufalin." Biochemical Pharmacology 70(10): 1479-1486. |
|
[6]
|
Huang, W.-W., et al. (2012). "Bufalin induces G0/G1 phase arrest through inhibiting the levels of cyclin D, cyclin E, CDK2 and CDK4, and triggers apoptosis via mitochondrial signaling pathway in T24 human bladder cancer cells." Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis 732(1-2): 26-33. |
|
[7]
|
Jiang, B., et al. (2012). "Induction of cytochrome P450 3A by Shexiang Baoxin Pill and its main components." Chemico-Biological Interactions 195(2): 105-113. |
|
[8]
|
Bhuiyan, M. B. A., et al. (2003). "Study on mechanism of action of Chinese medicine Chan Su: dose-dependent biphasic production of nitric oxide in trophoblastic BeWo cells." Clinica Chimica Acta 330(1-2): 179-184. |
|
[9]
|
Ye, M., et al. (2004). "Novel cytotoxic bufadienolides derived from bufalin by microbial hydroxylation and their structure-activity relationships." The Journal of Steroid Biochemistry and Molecular Biology 91(1-2): 87-98. |
|
[10]
|
Yang, Z., et al. (2013). "Enhancement of skin permeation of bufalin by limonene via reservoir type transdermal patch: Formulation design and biopharmaceutical evaluation." International Journal of Pharmaceutics 447(1-2): 231-240. |
|
[11]
|
Zhang, Y., et al. (2008). "Simultaneous determination of three bufadienolides in rat plasma after intravenous administration of bufadienolides extract by ultra performance liquid chromatography electrospray ionization tandem mass spectrometry." Analytica Chimica Acta 610(2): 224-231. |
|
| 连结 |
 中药材图像数据库 中药材图像数据库
 中药标本数据库 中药标本数据库
|
 中药材图像数据库
中药材图像数据库
 中药标本数据库
中药标本数据库






