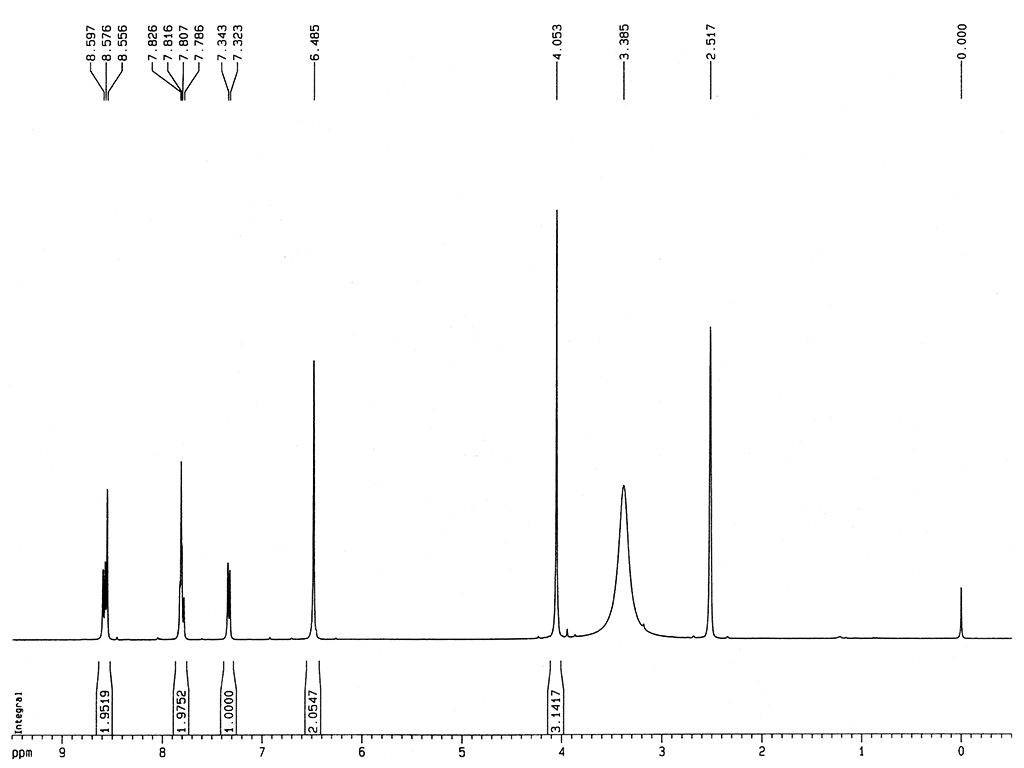
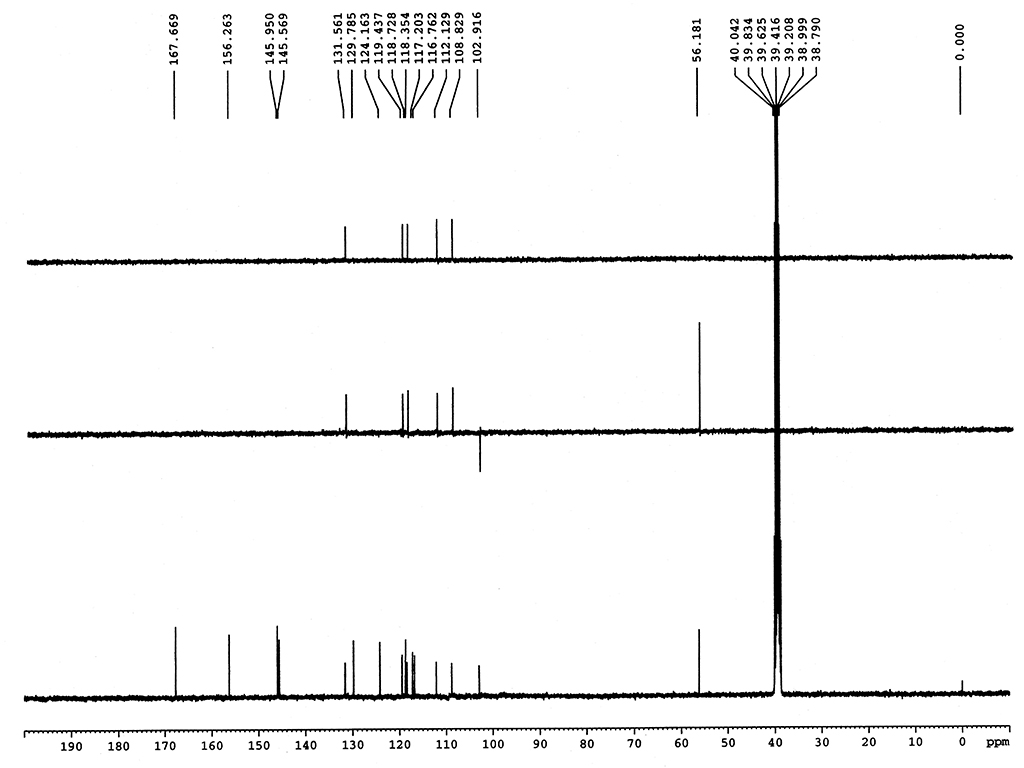
|
植物来源 |
|
|
生物活性 |
|
|
鉴定 |
熔点 |
281-286°C |
|
|
| 分析方法 |
|
| 仪器 |
RP-18F254板 (Merck, Darmstadt, Germany) |
| 流动相 |
2-丁酮: 甲醇: 硫酸钠 = 2: 1: 1 |
| 检测器 |
UV λ254 nm |
|
|
|
| 仪器 |
Agilent 3D CE 仪一个空气冷却和一个二极管数组检测器 (Agilent Technologies, Palo Alto, CA, USA) |
| 色谱柱 |
一个48.5 cm (40.0 cm to the detector) × 50 µm i.d. 未包膜硅胶融合毛细管 (Ruifeng Inc., Hebei, China); 10°C ; 30 kV |
| 流动相 |
1.0 M 氢氧化钠 (10 min), 水 (10 min) 和电泳缓冲液 (10 min) 轮流冲洗 |
| 检测器 |
UV λ254 nm |
|
|
|
| 仪器 |
Hitachi 液相色谱 (Tokyo, Japan) 配置一个 Model L-6200 泵 |
| 色谱柱 |
LiChrospher 100 RP-18反相色谱柱 (5 µm, 25 x 0.3 cm I.D., E. Merck) |
| 流动相 |
0.3% 碳酸铵溶液-乙腈混合溶剂 (75: 25, v/v) pH7.5, 0.8 mL/min |
| 检测器 |
UV λ254 nm |
|
|
|
| 仪器 |
高校液相色谱 (Waters 600 series泵及控制器) 接一个四极离子阱质谱仪 (ThermoFinnigan LCQ Classic) |
| 色谱柱 |
250 × 4.6 mm i.d., 5 µm, C18 色谱柱 (Supelco Discovery C18) |
| 流动相 |
A: 甲醇, B: 1% 乙酸溶液, 60-100% A 线性梯度10 min, 100%, 1 mL/min |
| 检测器 |
电喷雾 (ES) 源及大气压化学电离 (APCI) 源. APCI 源: 1 mL/min; ES 源: 175 µL/min. APCI 蒸发器: 450°C, 毛细管: 150°C, 针电流: 5 µA.ES 锥电压: 4.2 kV; 毛细管: 220°C. 鞘及辅助氮气: 80 and 20 psi. |
|
| 样品制备 |
|
方法一 |
|
|
|
|
|
球星硅胶 (粒径 5 µm 及 40-75 µm, 孔径 10 nm, 表面积 300 m2 g-1) Fuji Silysia Chemical (Kasugai, Japan) |
|
|
120°C 干燥硅胶过夜, 50% 水湿润的氮气, 吸水24 小时, 增重 5-6%. 60 mL 无水甲苯, 加入6.4 mL 三氯十八烷基, 1.2 mL 3-氯丙基三氯硅烷和 20 mL 甲苯混合物并在氮气中剧烈搅拌 24 小时. 过滤并用无水甲苯, 二氯甲烷, 甲醇, 水和甲醇冲洗, 80°C 干燥过夜. 加入 N, N-二甲基甲酰胺和N, N-二甲基乙基胺置于 80°C, 24 小时. 过滤甲醇, 水和甲醇冲洗并 80°C 干燥12 小时 |
|
|
100 g 样品1 L (5 mM K2HPO4 溶于50% 甲醇) 超声提取 30 min, 2 次, 收集提取物 (2 L) 并上样 C18SAX SPE 暗盒 (20 g/ 60 mL) 并以适当溶剂洗脱以 C18 TDE 柱 HPLC 制备; (A) 0.2% 甲酸, (B) 乙腈. 0-30 min: 20-70% B; 20.0 mL/min; 260 nm. |
|
|
5 mL 样品进 C18 TDE 色谱柱。SPE 浓缩 (C18 SAX, 1 g/6 mL), SPE 洗脱剂先真空离心, 40°C |
|
|
方法二 |
|
|
样品粉末与 70% 甲醇溶液混合18 hr, 室温. 离心去出残留物. |
|
|
| 参考文献 |
|
[1]
|
Li, W., et al. (2004). "Rapid determination of aristolochic acid I and II in Aristolochia plants from different regions by β-cyclodextrin-modified capillary zone electrophoresis." Journal of Chromatography A 1049(1-2): 211-217. |
|
[2]
|
Chan, W., et al. (2006). "Differentiation of herbs linked to Chinese herb nephropathy from the liquid chromatographic determination of aristolochic acids." Analytica Chimica Acta 576(1): 112-116. |
|
[3]
|
Stiborová, M., et al. (2008). "Metabolic activation of carcinogenic aristolochic acid, a risk factor for Balkan endemic nephropathy." Mutation Research/Reviews in Mutation Research 658(1-2): 55-67. |
|
[4]
|
Chen, S.-M., et al. (2007). "Pharmacokinetics and nephrotoxicity of aristolochic acid in rabbits." Toxicon 50(2): 180-188. |
|
[5]
|
Lee, T.-Y., et al. (2002). "High-performance liquid chromatographic determination for aristolochic acid in medicinal plants and slimming products." Journal of Chromatography B 766(1): 169-174. |
|
[6]
|
Kite, G. C., et al. (2002). "Detecting aristolochic acids in herbal remedies by liquid chromatography/serial mass spectrometry." Rapid Communications in Mass Spectrometry 16(6): 585-590. |
|
[7]
|
Wei, J., et al. (2012). "A new reversed-phase or strong anion-exchange mixed-mode stationary phase based on polar-copolymerized approach and its application in the enrichment of aristolochic acids." Journal of Chromatography A 1246(0): 129-136. |
|
[8]
|
Ohno, T., et al. (2006). "Identification tests of aristolochic acid in crude drugs by reversed-phase TLC/scanning densitometry." J. Health Sci. 52(1): 78-81. |
|
| 连结 |
 中药材图像数据库 中药材图像数据库
 药用植物图像数据库 药用植物图像数据库
 中药标本数据库 中药标本数据库
|
 中药材图像数据库
中药材图像数据库
 药用植物图像数据库
药用植物图像数据库
 中药标本数据库
中药标本数据库