|
Natural Resources |
|
|
Bioactivities |
|
|
Identification |
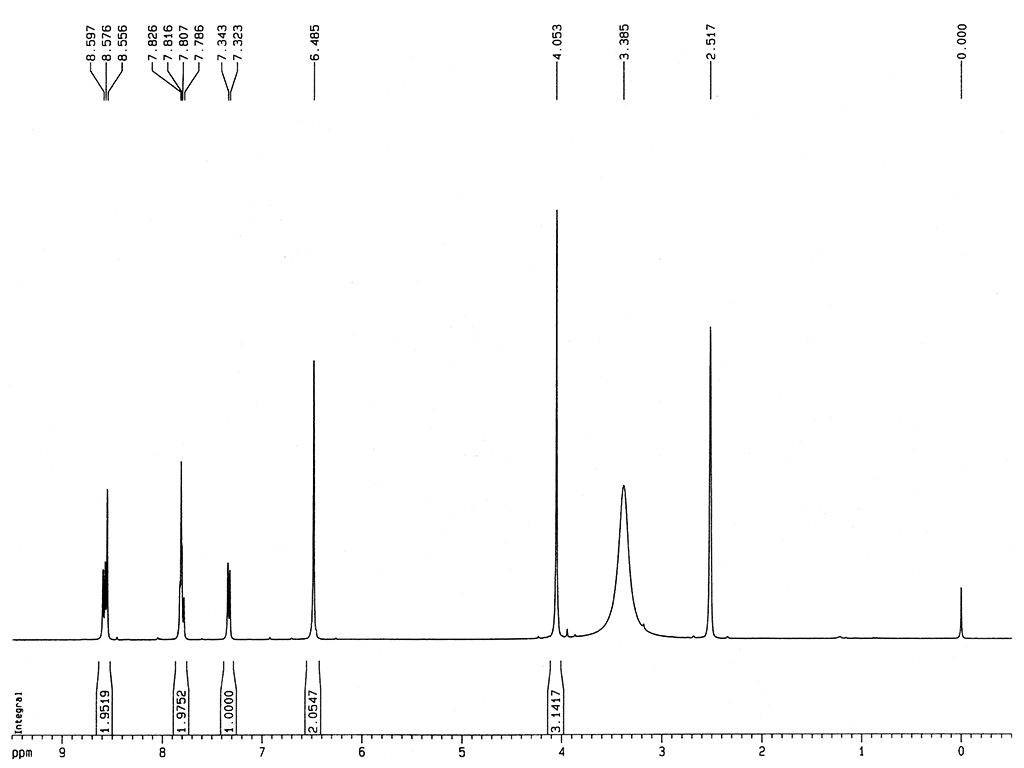
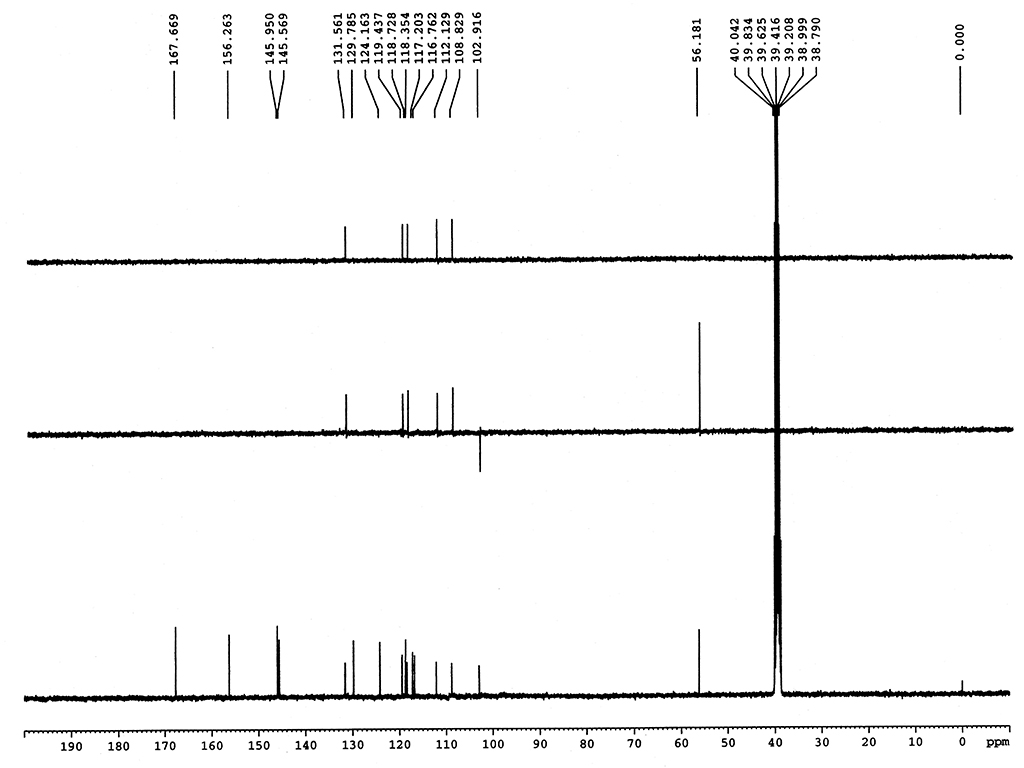
Melting point |
281-286°C |
|
|
| Analytical Method |
|
| INSTRUMENT |
RP-18F254 (Merck, Darmstadt, Germany) |
| MOBILE PHASE |
2-butanone: methanol: sodium sulfate = 2: 1: 1 |
| DETECTION |
UV λ254 nm |
|
|
|
| INSTRUMENT |
Agilent 3D CE system with air-cooling and a diode array detector (Agilent Technologies, Palo Alto, CA, USA) |
| COLUMN |
A 48.5 cm (40.0 cm to the detector) × 50 µm i.d. uncoated fused-silica capillary (Ruifeng Inc., Hebei, China); 10°C ; 30 kV |
| MOBILE PHASE |
Washed with1.0 M sodium hydroxide (10 min), water (10 min) and the running buffer (10 min) in turn |
| DETECTION |
UV λ254 nm |
|
|
|
| INSTRUMENT |
Hitachi liquid chromatograph (Tokyo, Japan) equipped with Model L-6200 pumps |
| COLUMN |
LiChrospher 100 RP-18 reversed-phase column (5 µm, 25 x 0.3 cm I.D., E. Merck) |
| MOBILE PHASE |
Mixed solvent of 0.3% ammonium carbonate solution-acetonitrile (75: 25, v/v) pH7.5, 0.8 mL/min |
| DETECTION |
UV λ254 nm |
|
|
|
| INSTRUMENT |
High performance liquid chromatograph (Waters 600 series pumps and controller) interfaced to a quadrupole ion-trap mass spectrometer (ThermoFinnigan LCQ Classic) |
| COLUMN |
250 × 4.6 mm i.d., 5 µm, C18 column (Supelco Discovery C18) |
| MOBILE PHASE |
A: Methanol, B: 1% aqueous acetic acid, Linear gradient of 60-100% A in 10 min followed by 100%, 1 mL/min |
| DETECTION |
Electrospray (ES) source and atmospheric pressure chemical ionisation (APCI) source. APCI source: 1 mL/min; ES source: 175 µL/min. APCI the vapouriser: 450°C, capillary: 150°C, needle current: 5 µA.ES cone voltage: 4.2 kV; capillary: 220°C. Sheath and auxiliary nitrogen gas: 80 and 20 psi. |
|
| Sample Preparation |
|
METHOD 1 |
|
|
|
|
|
Spherical silica (5 µm and 40-75 µm particle size, 10 nm pore size, 300 m2 g-1 surface area) Fuji Silysia Chemical (Kasugai, Japan) |
|
|
Silica gel was dried at 120°C overnight, absorb water by 50% water humidified nitrogen 24 hours to increase weight 5-6%. 60 mL of anhydrous toluene, mixture of 6.4 mL of trichlorooctadecylsilane, 1.2 mL of 3-chloropropyltrichlorosilane and 20 mL of toluene was added with vigorous stirring under nitrogen atmosphere for 24 hours. Filtered and washed with anhydrous toluene, dichloromethane, methanol, water and methanol and dry at 80°C overnight. N,N-dimethyl formamide and N,N-dimethylethylamine was added and placed at 80°C for 24 hours. Filtered and washed by methanol, water and methanol and dried at 80°C for 12 hours. |
|
|
100 g sample was sonicated in 1 L (5 mM K2HPO4 dissolved in 50% methanol) for 30 min twice, the extracts were combined (2 L) and loaded through a C18 SAX SPE cartridge (20 g/ 60 mL) and eluted with appropriate solution. C18 TDE column HPLC was used for preparation; (A) 0.2% formic acid, (B)acetonitrile. 0-30 min: 20-70% B; 20.0 mL/min; 260 nm. |
|
|
5 mL samples were injected into C18 TDE column. Concentrated fractions by SPE (C18 SAX, 1 g/6 mL), eluents from SPE were first dried by vacuum centrifuge at 40°C |
|
|
METHOD 2 |
|
|
Sample powder was mixed with 70% aqueous methanol and left for 18 hours at room temperature. The residue was removed by centrifugation. |
|
|
| Reference |
|
[1]
|
Li, W., et al. (2004). "Rapid determination of aristolochic acid I and II in Aristolochia plants from different regions by β-cyclodextrin-modified capillary zone electrophoresis." Journal of Chromatography A 1049(1-2): 211-217. |
|
[2]
|
Chan, W., et al. (2006). "Differentiation of herbs linked to Chinese herb nephropathy from the liquid chromatographic determination of aristolochic acids." Analytica Chimica Acta 576(1): 112-116. |
|
[3]
|
Stiborová, M., et al. (2008). "Metabolic activation of carcinogenic aristolochic acid, a risk factor for Balkan endemic nephropathy." Mutation Research/Reviews in Mutation Research 658(1-2): 55-67. |
|
[4]
|
Chen, S.-M., et al. (2007). "Pharmacokinetics and nephrotoxicity of aristolochic acid in rabbits." Toxicon 50(2): 180-188. |
|
[5]
|
Lee, T.-Y., et al. (2002). "High-performance liquid chromatographic determination for aristolochic acid in medicinal plants and slimming products." Journal of Chromatography B 766(1): 169-174. |
|
[6]
|
Kite, G. C., et al. (2002). "Detecting aristolochic acids in herbal remedies by liquid chromatography/serial mass spectrometry." Rapid Communications in Mass Spectrometry 16(6): 585-590. |
|
[7]
|
Wei, J., et al. (2012). "A new reversed-phase or strong anion-exchange mixed-mode stationary phase based on polar-copolymerized approach and its application in the enrichment of aristolochic acids." Journal of Chromatography A 1246(0): 129-136. |
|
[8]
|
Ohno, T., et al. (2006). "Identification tests of aristolochic acid in crude drugs by reversed-phase TLC/scanning densitometry." J. Health Sci. 52(1): 78-81. |
|
| Link to |
 Chinese Medicinal Material Images Database Chinese Medicinal Material Images Database
 Medicinal Plant Images Database Medicinal Plant Images Database
 Chinese Medicine Specimen Database Chinese Medicine Specimen Database
|
 Chinese Medicinal Material Images Database
Chinese Medicinal Material Images Database
 Medicinal Plant Images Database
Medicinal Plant Images Database
 Chinese Medicine Specimen Database
Chinese Medicine Specimen Database