|
Natural Resources |
|
|
Bioactivities |
|
|
Identification |
Melting point |
192-195°C |
| Optical rotation |
[α]20D-13.4 (c, 3.65 in DMF) |
1HNMR

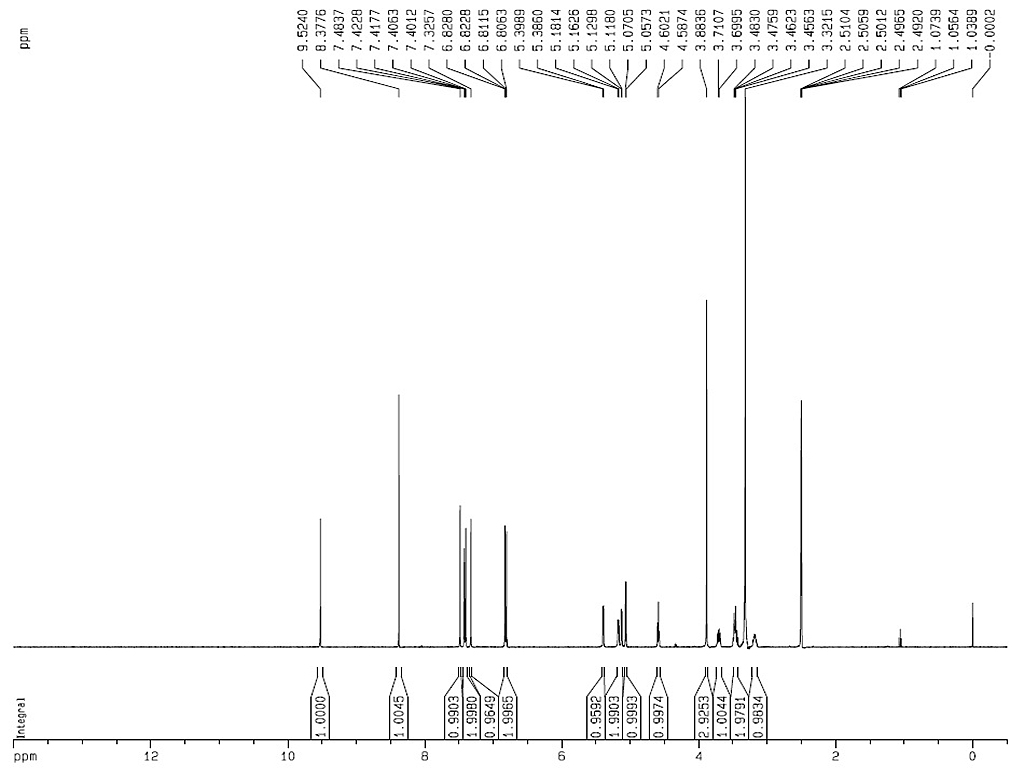
|
| Analytical Method |
|
| INSTRUMENT |
Silica plate GF254 |
| MOBILE PHASE |
Chloroform: ethyl acetate: methanol: concentrated ammonia = 10: 2: 2.5: 0.5 |
| DETECTION |
UV λ365 nm; 10% sulfuric acid in ethanol, heat up to 105°C |
|
|
|
| INSTRUMENT |
Hewlett Packard series 1100 HPLC (Hewlett Packard, palo alto, CA, USA) with a binary pump delivery system (G1322A) and a diode array detector (G1315A) |
| COLUMN |
Waters C18 column (Hypersil ODS, 4.6 × 250 mm, 5 µM, Wexford, Ireland) |
| MOBILE PHASE |
A: 1% acetic acid in water (v/v), B: acetonitrile, 0-40 min 10-20%, 40-70 min 20-100%, 0.8 mL/min |
| DETECTION |
UV λ260 nm |
|
|
|
| INSTRUMENT |
PU-980 Intelligent HPLC pump with a DG-980-50-3-line degasser, a LG-980-02 ternary gradient unit, and a MD-1510 multiwavelength detector (Jasco, Gross-Umstadt, Germany) |
| COLUMN |
Luna C18 (2) column (Phenomenexm, Aschaffenburg, Germany, 5 µm, 250 mm × 4.6 mm with guard column) |
| MOBILE PHASE |
A: 0.1% formic acid, B: CAN, 0-30 min 85% A, 30-40 min 76% A, 40-45 min 10% A, 45 min 85% A, 0.8 mL/min |
| DETECTION |
UV λ260 nm |
|
|
|
| INSTRUMENT |
UPLC™ system (Waters Acquity System, Milford, MA, USA) consists of a binary pump and an auto-sampler with a diode array detector (Waters 2996, Milford, MA) |
| COLUMN |
Reversed-phase Acquity UPLCTM EH C18 1.7 µm column (2.1 × 50 mm) (Waters Corp., Milford, MA, USA), 35°C |
| MOBILE PHASE |
A: ultra pure water with 0.1% formic acid, B: acetonitrile with 0.1% formic acid, 0-1min 10% B, 1-3 min 12% B, 3-4 min 22% B, 4-5 min 23% B, 5-6 min 35% B, 6-8 min 50%, 8-8.1 min 50% B, 8.1 min 10% B, 0.6 mL/min |
| DETECTION |
UV λ262 nm |
|
|
|
| INSTRUMENT |
A system consists of two pumps (LC-10ADvp, Shimadzu, Toyoto, Japan), an online degasser (DGU-12A, Shi-madzu), and triple quadrupole mass spectrometers with an ESI source (TSQ Quantum, Thermo Scientific, San Jose, CA). Both the liquid chromatograph and the mass spectrometer are controlled by Xcalibur software (Thermo Finnigan) |
| COLUMN |
Reversed-phase column (C18, 150 mm × 2.1 mm, 5 µm) |
| MOBILE PHASE |
A: 10% acetonitrile/ 0.1% formic acid, B: 90% acetonitrile/ 0.1% formic acid, 0-20 min 10-45% B; 20-25 min 100% A; 0.25 mL/min |
| DETECTION |
MS detector in positive mode, spray voltage: 3 kV, vaporizer temperature: 300°C, tube lens voltage: 150 V; capillary voltage: 35 V, capillary temperature: 270°C, sheath and aux gas flow rates: 35 and 10; optimized relative collision energies for collision-induced dissociation (CID): 30 V, m/z 255→199 for daidzein, m/z 271→153 for genistein, m/z 285→242 for glycitein and calycosin (IS), m/z 417→255 for daidzin, m/z 433→271 for genistin, and m/z 447→285 for glycitin SRM detection |
|
| Sample Preparation |
|
METHOD 1 |
|
|
Defat toasted soy flour twice with hexane. Extract the dried flour with 75% aqueous methanol. Evaporate the extract under vacuo and freeze-dry the raw extract. Clean and enrich the extract by SPE usingAmberlite XAD-7 material (Fluka, Buchs, Switzerland) in a glass column (45 cm × 65 cm). Apply the filtered aqueous crude extract. Wash the resin with water to remove proteins, sugars, organic acids, and salts and then elute it with methanol. Concentrate the eluate in vacuo. Dilute the eluate with water, and freeze-dry it to give an isoflavone-enriched XAD-7 extract. Apply the extract to HSCCC with MTBE/ ACN/ water (2/2/3 v/v/v) as mobile phase at flow ratre of 3.2 mL/min. The apparatus runs at 600 rpm and detection is UV λ260 nm. Obtain three major fractions. Apply fraction 1 for second HSCCC separation, using a \"one-step gradient solvent system\" to separate daidzin and glycitin. |
|
|
HSCCC made by Pharma-Tech Research (Baltimore, Maryland, USA) with three preparative coils, connected in series (total volume approximately 850 mL, id 2.6 mm), Biotronik HPLC pump BT 3020, Knauer Variable Wavelength Monitor |
|
|
Initial biphasic solvent system: MTBE/n-butanol/ACN/water (2/1/2/5 v/v/v/v), the lower phase is mobile phase; lower mobile phase after 280 min: MTBE-saturated water and ACN (10/1 v/v) |
|
|
2.5 mL/min; 600 rpm |
|
|
UV λ315 nm |
|
|
METHOD 2 |
|
|
Extract dried soybeans powder with 70% ethanol three times at 60°C. Evaporate ethanol under vacuum in a rotary evaporator to get ethanol extract. Dissolve the ethanol extract in distilled water. Partition the extract thrice with chloroform in a ratio of 1: 1. Then partition the ethanol-water fraction thrice with butanol in a ratio of 1: 1. Dry the butanol fraction under vacuum. |
|
|
Fractionate the butanol extract in a column packed with Sephadex LH-20 (100 M, Pharmacia Fine Chemical Co., Ltd., Germany) and elute it with 70% ethanol to give 20 fractions (F1-F20). Load each fraction of F7-F16 on a Sephadex LH-20 column. Use pure ethanol as eluting agent. |
|
|
Purify these isoflavones or glycosides on a C18 HPLC preparative column (Hypersil ODS, 250 × 22 mm, 10 m, Alltech, Deerfield, IL, USA) and elute it with 10% acetonitrile in 1% acetic acid solution. Purify seven isoflavones or glycosides, including glycitin. Evaporate each fraction to dryness and dissolve each in ethanol. |
|
|
METHOD 3 |
|
|
Extract Semen sojae praeparatum powder by SFE to remove oil. Reflux the powder with 70% aqueous EtOH. Carry out two additional extractions and the mix the extracts. Concentrate the extracts to remove EtOH. Charge the aqueous solution on D101 macroporous resin. Elute the column with water and then with 40%, 75% and 95% EtOH. |
|
|
Preparative HPLC (pHPLC) is carried out for separation. System: Waters 515 pump, 2487 detector, and N2000 workstation (Zhejiang University, Hangzhou, China); column: YWG C18 (20.0 × 250 mm, i.d. 10 μm) (Hanbon Science and Technology CO., Ltd. Jiang Su province, China); mobile phases: MeCN-water-AcOH (25: 75: 2 and 35: 65: 2 v/v/v); flow rate: 3.5 and 4.5 mL/min; detection: 260 nm |
|
|
| Reference |
|
[1]
|
Lee, C. H., et al. (2005). "Relative antioxidant activity of soybean isoflavones and their glycosides." Food Chemistry 90(4): 735-741. |
|
[2]
|
Shi, S., et al. (2012). "Systematic separation and purification of 18 antioxidants from Pueraria lobata flower using HSCCC target-guided by DPPH-HPLC experiment." Separation and Purification Technology 89(0): 225-233. |
|
[3]
|
Lapčík, O., et al. (2005). "Identification of isoflavones in Acca sellowiana and two Psidium species (Myrtaceae)." Biochemical Systematics and Ecology 33(10): 983-992. |
|
[4]
|
Li, X., et al. (2009). "Isoflavone glycosides from the bark of Maackia amurensis." Yaoxue Xuebao 44(1): 63-68. |
|
[5]
|
Pandey, A., et al. (2011). "Pharmacological screening of Coriandrum sativum Linn. for hepatoprotective activity." J Pharm Bioallied Sci 3(3): 435-441. |
|
[6]
|
Lee, E.-J., et al. (2010). "Glycitein inhibits glioma cell invasion through down-regulation of MMP-3 and MMP-9 gene expression." Chemico-Biological Interactions 185(1): 18-24. |
|
[7]
|
Roland, W. S., et al. (2011). "Soy isoflavones and other isoflavonoids activate the human bitter taste receptors hTAS2R14 and hTAS2R39." J Agric Food Chem 59(21): 11764-11771. |
|
[8]
|
Muñoz, Y., et al. (2009). "Equol is more active than soy isoflavone itself to compete for binding to thromboxane A2 receptor in human platelets." Thrombosis Research 123(5): 740-744. |
|
[9]
|
Wang, Z.-s., et al. (2012). "Study on the quality standard of peigen capsules." Zhongguo Yaofang 23(7): 635-637. |
|
[10]
|
Stürtz, M., et al. (2006). "Preparative isolation of isoflavones from soy and red clover." Molecular Nutrition & Food Research 50(4-5): 356-361. |
|
[11]
|
Toro-Funes, N., et al. (2012). "Fast simultaneous determination of free and conjugated isoflavones in soy milk by UPLC." Food Chemistry 135(4): 2832-2838. |
|
[12]
|
Qing, L.-S., et al. (2013). "Rapid Magnetic Solid-Phase Extraction for the Selective Determination of Isoflavones in Soymilk Using Baicalin-Functionalized Magnetic Nanoparticles." Journal of Agricultural and Food Chemistry 61(34): 8072-8078. |
|
[13]
|
Qu, L.-p., et al. (2007). "Isolation of six isoflavones from Semen sojae praeparatum by preparative HPLC." Fitoterapia 78(3): 200-204. |
|
| Link to |
 Chinese Medicinal Material Images Database Chinese Medicinal Material Images Database
 Medicinal Plant Images Database Medicinal Plant Images Database
|
 Chinese Medicinal Material Images Database
Chinese Medicinal Material Images Database
 Medicinal Plant Images Database
Medicinal Plant Images Database


