|
Natural Resources |
|
|
Bioactivities |
|
|
Identification |
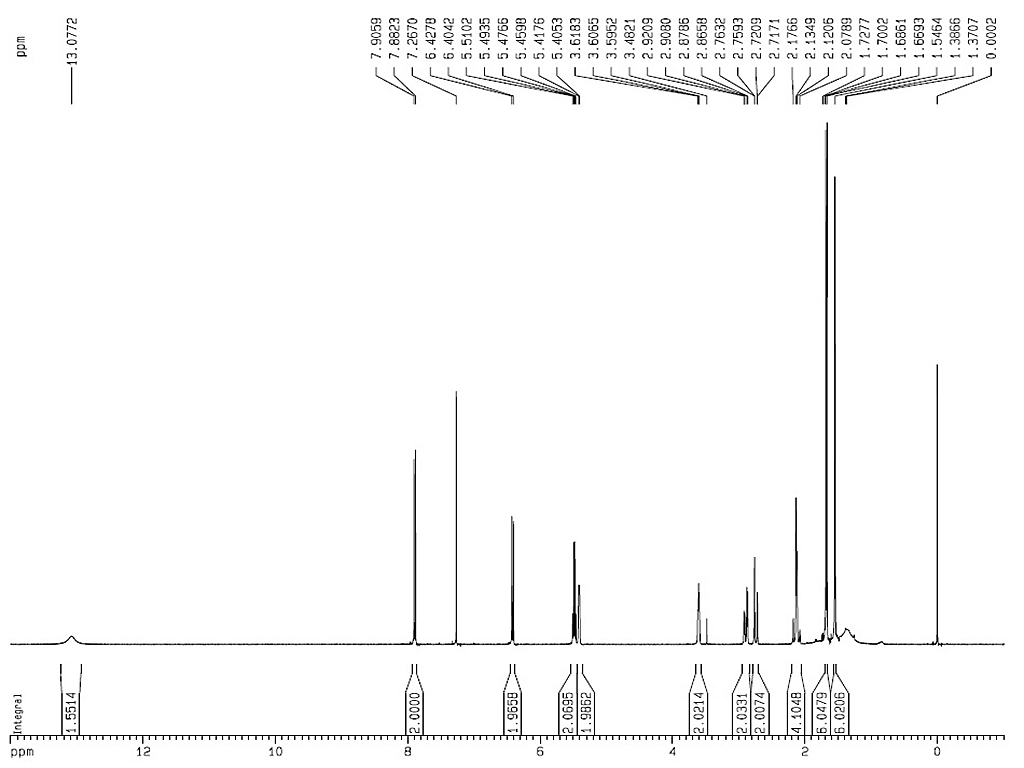
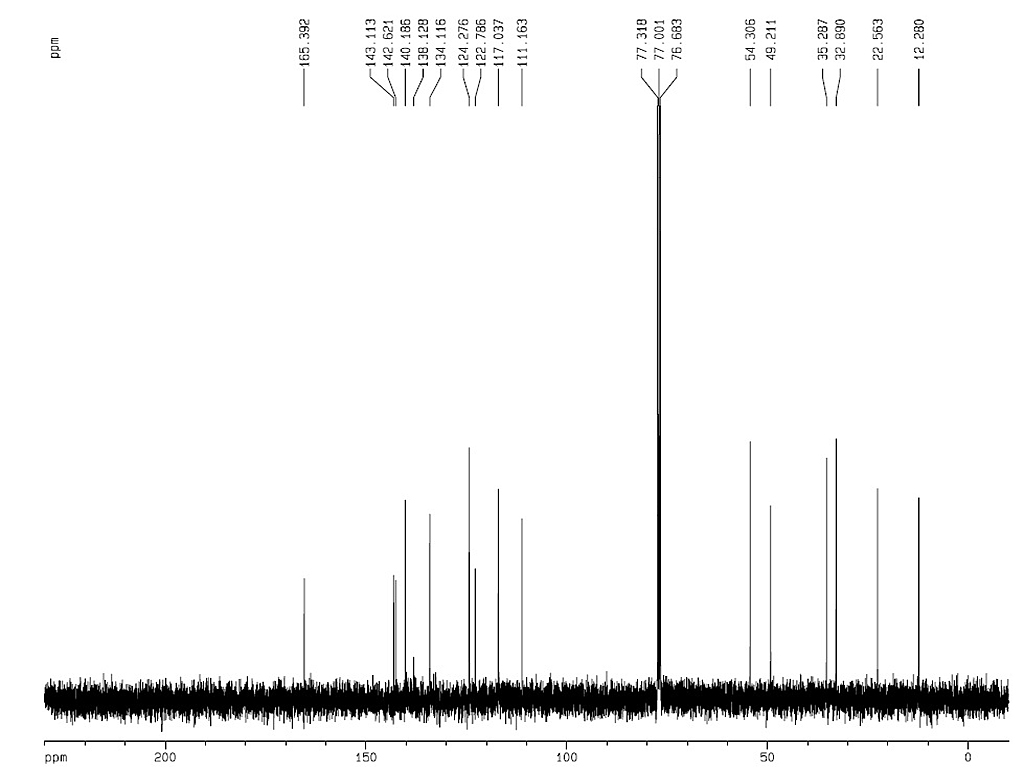
Melting point |
230°C |
| Optical rotation |
[α]24.5D-150.4 (c, 0.498 in MeOH)
[α]D-99 |
|
|
| Analytical Method |
|
| INSTRUMENT |
Agilent 1100 model (Agilent Technologies, Palo Alto, CA), having an integrated diode-array detector, an injector loop, and an autosampler. |
| COLUMN |
YMCBasic C18 column, 250 × 4.6 mm i.d., 5 μm (Waters Associates, Milford, MA), column temperature, 30°C |
| MOBILE PHASE |
A: 10 mM ammonium acetate pH = 3.5 adjusted with acetic acid), B: 100% methanol, 0-10 min 5-28% B, 10-20 min 100% B, 20.1-25 min 5% B, 1 mL/min |
| DETECTION |
A Finnigan LCQ™ DUO instrument (ThermoQuest Finnigan, San Jose, CA) equipped with an atmospheric pressure ionization (API) source and an electrospray ionization (ESI) inlet. UV λ200- 500 nm. Positive-ion mode, sheath gas flow rate, 80 arbitrary units; auxiliary gas flow rate, 25 arbitrary units; spray voltage, 4.8 kV; capillary temperature, 250°C ; and capillary voltage, 20 V. |
|
|
|
| INSTRUMENT |
Shimadzu LC-20AT system with two LC-20AT solvent delivery units, a SPD-M20A DAD detector, a SIL-20A auto sampler, a CTO-10ASVP column oven |
| COLUMN |
Analytical reversed phase C18 column (4.6 mm × 250 mm, 5 μm, Diamodsil™), 30°C |
| MOBILE PHASE |
Methanol : 0.1% (v/v) TFA in water = 18 : 82 (v/v), 1 mL/min |
| DETECTION |
UV λ308 nm |
|
|
|
| INSTRUMENT |
Shimadzu HPLC equipment consisted of two LC-10ADVP pumps, one RF-10AXL fluorescence detector, a manual 7725i injection with a 20 μl fixed loop, a CTO-10AVP column oven, a SCL-10VP system controller and a CLASS-VP HPLC work station. |
| COLUMN |
Analytical column was a Kromasil ODS C8 (150 mm × 4.6 mm, 5 μm), 40°C |
| MOBILE PHASE |
Methanol: water: triethanol amine = 45: 55: 0.05 v/v/v, 1.0 mL/min. |
| DETECTION |
Fluorescence detector has excitation at UV λ310 nm and emission at UV λ370 nm |
|
|
|
| INSTRUMENT |
HPLC system (Agilent 1100 series) consisted of a binary pump, an autosampler (20 μl injection) |
| COLUMN |
Nucleosil C18 column (5 μm, 50mm × 4.6 mm i.d. from Dalian Johnsson Separation Science and Technology Corp., Dalien, China) |
| MOBILE PHASE |
Acetonitrile: methanol: 10 mM ammonium acetate = 35: 40: 25, v/v/v, 0.40 mL/min |
| DETECTION |
Applied Biosystems Sciex API 4000 mass spectrometer (Applied Biosystems Sciex, Ontario, Canada) using TurboIonSpray for ion production. Positive ion mode (ion spray voltage 5000 V) with nitrogen as nebulizing (gas 1), heater (gas 2), curtain and collision gas (five units). High-flow gas flow parameters were optimized (nebulizer 40, heater 50 and curtain 10 units), 0.4 mL/min. The TurboIonSpray: 500°C, declustering potential: 40 V, collision energy: 35 V, collision cell exit: 10 V. |
|
| Sample Preparation |
|
METHOD 1 |
|
|
The smashed powder was added into hydrochloric acid solution (Solid/liquid = 1/10 (w/v), pH 2.0), executed for 20 hours by ultrasonication at room temperature. After two times extraction, acid filter solution was mixed together and adjusted to 9.0 by aqueous ammonia. |
|
|
Using a low-pressure glass chromatographic column (35 mm × 500 mm) filled with pretreated SP850 macroporous resin (Diaion High Porous Polymer SP-series, 0.315-1.25 mm particle size, Mitsubishi Chemical Industries Limited, Tokyo, Japan). Extract solution (10 L, pH 9.0) was loaded onto the column and adsorbed at a flow rate of 5 BV/h. After adsorption, washed the column with 6 BV deionized water, and eluted with 3 BV of 10% (v/v) ethanol solution to remove the polar impurities. Eluted with 6 BV of 70% (v/v) ethanol solution at 5 BV/h. At last, the non-polar contaminants were eluted with 3 BV of 100% ethanol. |
|
|
A glass chromatographic column (30 mm × 800 mm, H&E Co., Ltd. Beijing) filled with YMC C18 packing (50 μm particle size, Greenherbs Science & Technology Department Corporation, Ltd.). Extract was dissolved in 20% ethanol, 20% (v/v) ethanol was used to elute for the first 60 min, then 30% (v/v) ethanol was used to elute the products for the next 60 min, at last, 100% (v/v) ethanol was used to wash the impurity left on the column for 60 min. |
|
|
Preparative HPLC was performed by Waters Prep 4000 liquid chromatography system equipped with a fluid handling unit (pump heads), controller (for solvent gradient, flow rate, external events, and sparging process), a 2487 dual-wavelength absorbance detector with a preparative cell (Waters, Milford, MA), an Empower workstation (Waters, USA) and a reversed phase C18 column (19 mm × 300 mm, 7 μm, Symmetry Prep™). A: 0.1% (v/v) TFA in water, B: methanol, 0-20 min 15% B, 20-30 min 15-35% B, 30-40 min 35% B, 8 mL/min, UV λ308 nm. |
|
|
| Reference |
|
[1]
|
Zhang, H., et al. (2012). "Simultaneously preparative purification of Huperzine A and Huperzine B from Huperzia serrata by macroporous resin and preparative high performance liquid chromatography." Journal of Chromatography B 904(0): 65-72. |
|
[2]
|
Wang, Z. F. and X. C. Tang (2007). "Huperzine A protects C6 rat glioma cells against oxygen-glucose deprivation-induced injury." FEBS Letters 581(4): 596-602. |
|
[3]
|
Zhou, J., et al. (2001). "Huperzine A and donepezil protect rat pheochromocytoma cells against oxygen-glucose deprivation." Neuroscience Letters 306(1-2): 53-56. |
|
[4]
|
Haigh, J. R., et al. (2008). "Protection of red blood cell acetylcholinesterase by oral huperzine A against ex vivo soman exposure: Next generation prophylaxis and sequestering of acetylcholinesterase over butyrylcholinesterase." Chemico-Biological Interactions 175(1-3): 380-386. |
|
[5]
|
Wang, Z.-f., et al. (2006). "Effects of huperzine A on memory deficits and neurotrophic factors production after transient cerebral ischemia and reperfusion in mice." Pharmacology Biochemistry and Behavior 83(4): 603-611. |
|
[6]
|
Cheng, D. H. and X. C. Tang (1998). "Comparative Studies of Huperzine A, E2020, and Tacrine on Behavior and Cholinesterase Activities." Pharmacology Biochemistry and Behavior 60(2): 377-386. |
|
[7]
|
Wang, L. M., et al. (2000). "Huperzine A improves cognitive deficits caused by chronic cerebral hypoperfusion in rats." European Journal of Pharmacology 398(1): 65-72. |
|
[8]
|
Wang, L. S., et al. (2002). "Huperzine A attenuates cognitive deficits and brain injury in neonatal rats after hypoxia-ischemia." Brain Research 949(1-2): 162-170. |
|
[9]
|
Patel, P. A., et al. (2013). "Comparative in vitro and in vivo evaluation of lipid based nanocarriers of Huperzine A." International Journal of Pharmaceutics 446(1-2): 16-23. |
|
[10]
|
Peng, Y., et al. (2007). "Huperzine A regulates amyloid precursor protein processing via protein kinase C and mitogen-activated protein kinase pathways in neuroblastoma SK-N-SH cells over-expressing wild type human amyloid precursor protein 695." Neuroscience 150(2): 386-395. |
|
[11]
|
Yang, Q. P., et al. (2003). "Determination of huperzine A in formulated products by reversed-phase-liquid chromatography using diode array and electrospray ionization mass spectrometric detection." Phytomedicine 10(2-3): 200-205. |
|
[12]
|
Zhang, H., et al. (2012). "Simultaneously preparative purification of Huperzine A and Huperzine B from Huperzia serrata by macroporous resin and preparative high performance liquid chromatography." Journal of Chromatography B 904(0): 65-72. |
|
[13]
|
Yue, P., et al. (2007). "Determination of Huperzine A in rat plasma by high-performance liquid chromatography with a fluorescence detector." Journal of Pharmaceutical and Biomedical Analysis 44(1): 309-312. |
|
[14]
|
Wang, Y., et al. (2004). "Liquid chromatographic-tandem mass spectrometric method for the quantitation of huperzine A in dog plasma." Journal of Chromatography B 803(2): 375-378. |
|
| Link to |
 Medicinal Plant Images Database Medicinal Plant Images Database
|
 Medicinal Plant Images Database
Medicinal Plant Images Database