|
Natural Resources |
|
|
Bioactivities |
|
|
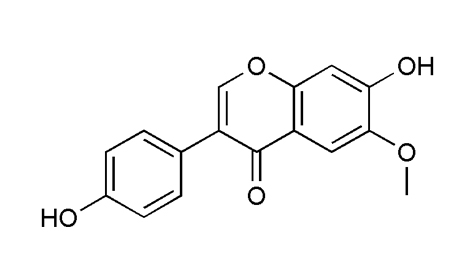
Identification |
Melting point |
337-339°C |
1HNMR

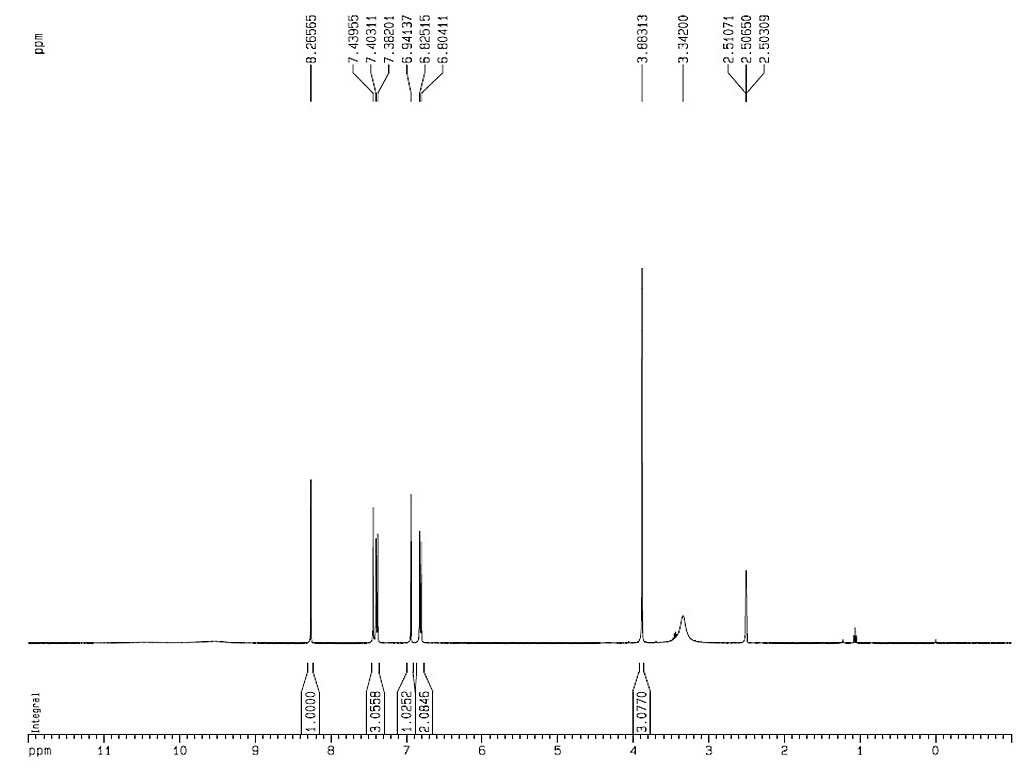
|
| Analytical Method |
|
| INSTRUMENT |
HSGF254 plates (Yantai Chemical Engineering Institute, China) |
| MOBILE PHASE |
Toluene: ethyl acetate: acetone: formic acid = 20: 4: 2: 1 |
| DETECTION |
UV λ254 nm; fumes of concentrated ammonia solution |
|
|
|
| INSTRUMENT |
Shimadzu LC-2010AHT HPLC system and Shimadzu CLASS-VP software version 6.12 SP4 (Shimadzu Co. Ltd., Japan) |
| COLUMN |
Kromasil C18 column (4.6 × 250 mm, 5 µm, Tianjin Scientific Instruments Co. Ltd., China), 40°C |
| MOBILE PHASE |
A: 0.5% glacial acetic acid in water, B: 0.5% glacial acetic acid in methanol, 0-10 min 30% B, 10-30 min 30-40% B, 30-40 min 40% B, 40-60 min 40-60% B, injection volume: 25 µL, 1.0 mL/min |
| DETECTION |
UV λ257.2 nm |
|
|
|
| INSTRUMENT |
Agilent 1200 reversed-phase HPLC system |
| COLUMN |
HiQ Sil C18W reversed-phase column (250 mm × 4.6 mm i.d., 5 µm), 30°C |
| MOBILE PHASE |
Methanol: water: formic acid = 35: 64.935: 0.065 (v/v/v), 1 mL/min, injection volume: 10 µl |
| DETECTION |
UV λ260 nm |
|
|
|
| INSTRUMENT |
HP 1200 Series chromatograph from Agilent (Waldbronn, Germany) equipped with a binary pump, a membrane degasser, an autosampler, and a six-port valve. |
| COLUMN |
Zorbax Eclipse XDB-C18 (50 × 4.6 mm2, 1.8 µm), 25°C |
| MOBILE PHASE |
A: acetonitrile, B: 0.01% aqueous formic acid, 0-1.5 min 10% A, 1.5-2.5 min 10-25% A, 2.5-3.5 min 25% A, 3.5-7 min 25-50% A, 7-8 min 50-80% A, 8-10 min 80% A, 10-12 min 80-10% A, 0.5 mL/min, injection volume: 10 µL |
| DETECTION |
Triple Quad LC/MS 6410 (Agilent) equipped with an electrospray (ESI) source. ESI-MS spectra were acquired in positive-ion multiple reaction monitoring (MRM) mode, electrospray capillary voltage: 3500 V, nebulizer pressure: 35 psi. drying gas and flow rate: nitrogen 12 L/min at 350°C. The whole system was controlled by an Agilent Mass Hunter software, version B.04.01. |
|
| Sample Preparation |
|
METHOD 1 |
|
|
|
|
|
Type-J multilayer coil planet centrifuge (P.C. Inc., Potomac, MD, USA). It holds a column holder and a counterweight in the symmetrical positions at a distance of 10 cm from the central axis of the centrifuge. The separation column was prepared by winding a single piece of Tefzel tubing (Zeus Industrial Products) of 1.6 mm I.D. (SW 14), 160 m in length around the column holder hub making 12 layers between a pair of flanges spaced 2 inches apart. The total capacity of the column is about 320 ml. The β-values varied from 0.5 at the internal terminal to 0.75 at the external terminal. The revolution speed of the apparatus was regulated with a controller (Bodine Electric, North Chicago, IL, USA) where an optimum 800 rpm was used in the present studies. A UV monitor (Uvicord S, LKB Instruments, Bromma/Stockholm, Sweden). A fraction collector (Ultrorac, LKB Instruments). |
|
|
Lower phase of HCl3-MeOH-H2O (4: 3: 2, v/v) |
|
|
2.0 mL/min; 800 rpm |
|
|
UV λ275 nm |
|
|
METHOD 2 |
|
|
Negative pressure cavitation equipment (CN2597047) was developed in our laboratory. The schematic diagram of the device is shown in Fig. 2A. The negative pressure cavitation device consists of an extraction pot (1) and a collection pot (2). Material and solvent are added into the extraction pot through the inlet (3). The negative pressure of the device is provided by a vacuum pump through an interface on top of the pot body. Nitrogen is introduced into the extraction pot through valve 2. After extraction, the extraction solvent is filtered through a net (4) into the collection pot. The residue can be removed from the pot through the discharge lid. The temperature of the entire system can be kept constant because nitrogen with ambient temperature is injected into the system continuously. The volatilized solvent is refrigerated by the condenser (6). |
|
|
Ten grams of pigeon pea roots were introduced into the NPCE device from the sample portal. After the solvent was added, the device was connected to the vacuum pump. At the same time, the valve of flow meter was opened and nitrogen was supplied into the device. The negative pressure of the device can be controlled by the valve. According to the experimental design, the extraction process was performed under different conditions. The subsequent process was the same as ME. The effects of extraction time, particle size, negative pressure, ethanol concentration and liquid/solid ratio on the extraction efficiency of two isoflavonoids were investigated. |
|
|
| Reference |
|
[1]
|
Zhang, D.-Y., et al. (2010). "Negative pressure cavitation extraction and antioxidant activity of genistein and genistin from the roots of pigeon pea [Cajanus cajan (L.) Millsp.]." Separation and Purification Technology 74(2): 261-270. |
|
[2]
|
Shodehinde, S. A. and G. Oboh (2013). "Antioxidant properties of aqueous extracts of unripe Musa paradisiaca on sodium nitroprusside induced lipid peroxidation in rat pancreas in vitro." Asian Pacific Journal of Tropical Biomedicine 3(6): 449-457. |
|
[3]
|
Lee, J. H., et al. (2013). "Determination of the variations in levels of phenolic compounds in soybean (Glycine max Merr.) sprouts infected by anthracnose (Colletotrichum gloeosporioides)." J Sci Food Agric 93(12): 3081-3086. |
|
[4]
|
Kasper, J. and M. F. Melzig (2011). "HPTLC method for the quantification of isoflavones in nutritional supplements of red clover (Trifolium pratense L.)." J. Planar Chromatogr.--Mod. TLC 24(5): 373-375. |
|
[5]
|
Lengyel, J., et al. (2013). "On the radical scavenging activity of isoflavones: thermodynamics of O-H bond cleavage." Physical Chemistry Chemical Physics 15(26): 10895-10903. |
|
[6]
|
Vitale, D. C., et al. (2013). "Isoflavones: estrogenic activity, biological effect and bioavailability." Eur J Drug Metab Pharmacokinet 38(1): 15-25. |
|
[7]
|
Weng, C. J. and G. C. Yen (2012). "Flavonoids, a ubiquitous dietary phenolic subclass, exert extensive in vitro anti-invasive and in vivo anti-metastatic activities." Cancer Metastasis Rev 31(1-2): 323-351. |
|
[8]
|
Bae, M., et al. (2012). "Inhibitory effects of isoflavonoids on rat prostate testosterone 5alpha-reductase." J Acupunct Meridian Stud 5(6): 319-322. |
|
[9]
|
Hirohata, M., et al. (2012). "Anti-amyloidogenic effects of soybean isoflavones in vitro: Fluorescence spectroscopy demonstrating direct binding to Aβ monomers, oligomers and fibrils." Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 1822(8): 1316-1324. |
|
[10]
|
Masilamani, M., et al. (2012). "Regulation of the immune response by soybean isoflavones." Immunologic Research 54(1-3): 95-110. |
|
[11]
|
Kim, J. H., et al. (2012). "A new isoflavone glycitein 7-O-beta-D-glucoside 4\'-O-methylate, isolated from Cordyceps militaris grown on germinated soybeans extract, inhibits EGF-induced mucus hypersecretion in the human lung mucoepidermoid cells." Phytother Res 26(12): 1807-1812. |
|
[12]
|
Yuan, D., et al. (2006). "TLC and HPLC analysis of soy isoflavones in Semen Sojae Praeparatum." Asian J. Tradit. Med. 1(3-4): 166-172. |
|
[13]
|
Delgado-Zamarreño, M. M., et al. (2012). "A modified QuEChERS method as sample treatment before the determination of isoflavones in foods by ultra-performance liquid chromatography-triple quadrupole mass spectrometry." Talanta 100(0): 320-328. |
|
[14]
|
Yang, F., et al. (2001). "Separation and purification of isoflavones from a crude soybean extract by high-speed counter-current chromatography." Journal of Chromatography A 928(2): 163-170. |
|
| Link to |
 Chinese Medicinal Material Images Database Chinese Medicinal Material Images Database
 Medicinal Plant Images Database Medicinal Plant Images Database
|
 Chinese Medicinal Material Images Database
Chinese Medicinal Material Images Database
 Medicinal Plant Images Database
Medicinal Plant Images Database


