|
植物来源 |
|
|
异名 |
|
|
生物活性 |
|
|
鉴定 |
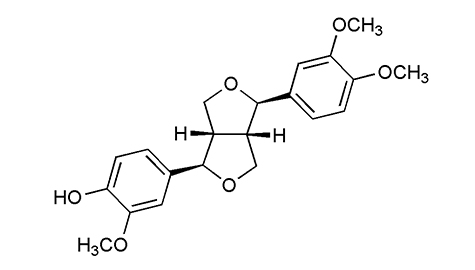
熔点 |
133-134°C |
| 旋亮度 |
[α]22D+120° (c, 0.04 甲醇)
[α]20D+158° (c, 0.5 甲醇) |
|
|
| 分析方法 |
|
| 仪器 |
Agilent 1200反相HPLC 系统 |
| 色谱柱 |
Kinetex 2.6 μm PFP 100A反相色谱柱 (100 mm × 4.6 mm i.d.), 25°C |
| 流动相 |
A: 甲醇; B: 0.1% 甲酸水, 0-10 min 20-35% A; 10-20 min 35% A; 20-25 min 35-40% A; 25-40 min 40-65% A; 1 mL/min |
| 检测器 |
UV λ270 nm |
|
|
|
| 仪器 |
Alliance 2690 HPLC 仪 (Waters, Milford, MA, USA) |
| 色谱柱 |
Waters Nova-Pak®C18 色谱柱 (3.9 × 150 mm) |
| 流动相 |
A: 3% 乙酸水, v/v, B: 乙腈, 系统1, 0-34min 90-83% A, 系统2, 0min 95% A, 15 min 30% A, 系统3, 0min 95% A, 50 min 30% A, 系统2: 0 min 95% A, 15 min 30% A, 系统3: 0min 95% A, 50min 30% A, 1 mL/min |
| 检测器 |
UV λ280 nm |
|
|
|
| 仪器 |
Agilent 1100 series HPLC 系统 (Agilent, San Jose, CA, USA) |
| 色谱柱 |
HiQ Sil C18 V 反相色谱柱 (250 mm × 4.6 mm I.D., 5 μm, Kya Tech, Hachioji City, Japan), 30°C |
| 流动相 |
A: 甲醇, B: 去离子水; 0-2 min 40% A; 3-4 min 40-60% A; 5-12 min 60-80% A; 12-13 min 80-40% A; 1.0 mL/min |
| 检测器 |
正离子模式; 离子喷雾电压: 3.8 kV; 毛细管温度: 260°C ; 毛细管电压40 V; 鞘气 35 (arbitrary units), 辅助气 10 (arbitrary units), 吹扫气 3 (arbitrary units); 管镜头120 V. |
|
| 样品制备 |
|
方法一 |
|
|
80% 丙酮提取 (质量: 容量 = 1: 8) |
|
|
大孔吸附树脂柱纯化, 乙醇梯度洗脱, 收集 70% 洗脱物冻干得到 0.2 g 提取物 (纯度> 98.6%). |
|
|
| 参考文献 |
|
[1]
|
Kuang, H.-X., et al. (2009). "A New Caffeoyl Phenylethanoid Glycoside from the Unripe Fruits of Forsythia suspensa." Chinese Journal of Natural Medicines 7(4): 278-282. |
|
[2]
|
Song, W., et al. (2012). "Interaction between phillygenin and human serum albumin based on spectroscopic and molecular docking." Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 85(1): 120-126. |
|
[3]
|
Jiao, J., et al. (2012). "Application of white-rot fungi treated Fructus forsythiae shell residue as a low-cost biosorbent to enrich forsythiaside and phillygenin." Chemical Engineering Science 74(0): 244-255. |
|
[4]
|
Miyazawa, M., et al. (1992). "Phenolic lignans from flower buds of Magnolia fargesii." Phytochemistry 31(10): 3666-3668. |
|
[5]
|
Rahman, M. M. A., et al. (1990). "Lignans of Forsythia intermedia." Phytochemistry 29(6): 1971-1980. |
|
[6]
|
Vidigal, M. C. S., et al. (1995). "Lignans from kernels of Virola michelii Heckel." Phytochemistry 40(4): 1259-1261. |
|
[7]
|
Jiao, J., et al. (2013). "Comparison of main bioactive compounds in tea infusions with different seasonal Forsythia suspensa leaves by liquid chromatography–tandem mass spectrometry and evaluation of antioxidant activity." Food Research International 53(2): 857-863. |
|
[8]
|
Xue, J., et al. (2010). "Triterpenoids from the Fruits of Forsythia suspensa." Chinese Journal of Natural Medicines 8(6): 414-418. |
|
[9]
|
Halls, S. C. and N. G. Lewis (2003). "Reversed-phase HPLC lignan chiral analysis with laser polarimetric detection." Tetrahedron: Asymmetry 14(6): 649-658. |
|
| 连结 |
 中药材图像数据库 中药材图像数据库
 药用植物图像数据库 药用植物图像数据库
 中药标本数据库 中药标本数据库
|
 中药材图像数据库
中药材图像数据库
 药用植物图像数据库
药用植物图像数据库
 中药标本数据库
中药标本数据库



