|
植物來源 |
|
|
生物活性 |
|
|
鑑定 |
熔點 |
240-242°C |
| 旋光度 |
[α]24D+44 (c, 1.03 50% 丙酮) |
1HNMR

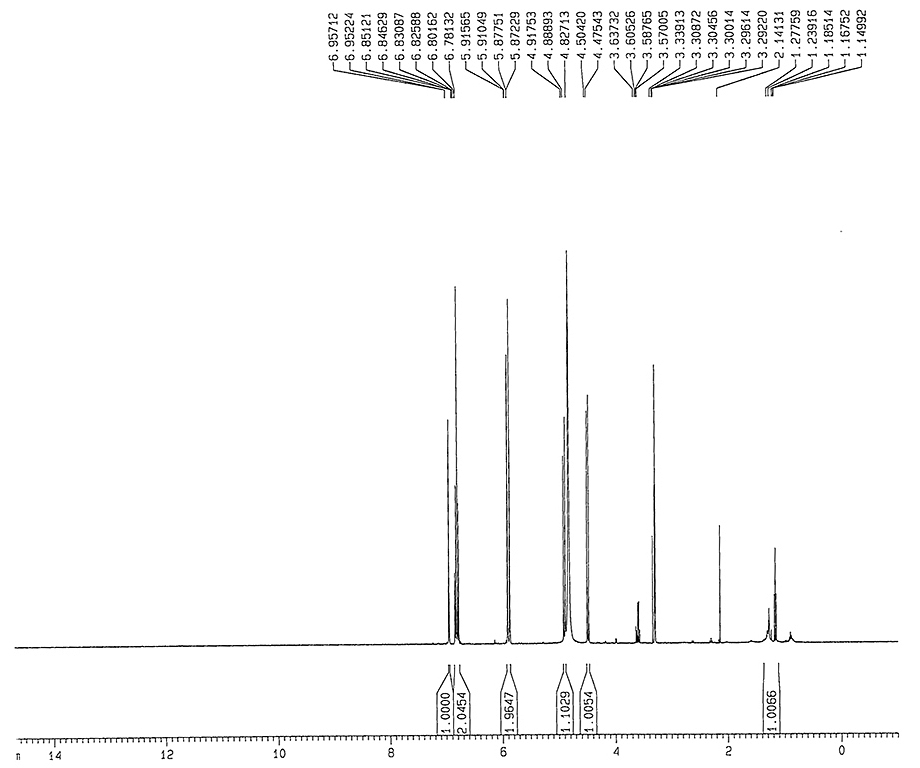
|
| 分析方法 |
|
| 儀器 |
Agilent-1100 HPLC儀 |
| 色譜柱 |
Agilent TC-C18 色譜柱 (4.6 mm x 250 mm, 5 µm), 25°C |
| 流動相 |
A: 1% 磷酸水, B: 甲醇: 乙腈 = 60: 40, 0-23 min 26-31% B, 23-25 min 31-38% B, 25-50 min 38-43% B, 1 mL/min |
| 檢測器 |
0-42 min UVλ290 nm, 42-49 min UVλ371 nm |
|
|
|
| 儀器 |
ACQUITY UPLC 系統 (Waters, Milford, MA, USA) 包括二元泵, 進樣控制器, 色譜柱控制器, PDA檢測器, 自動進樣溫度4°C. |
| 色譜柱 |
Agilent Zorbax Eclipse XDB 色譜柱 (150 mm × 2.1 mm i.d., 5 μm; Agilent Technologies, CA, USA), 35°C |
| 流動相 |
A: 1% 乙酸水溶液和甲醇90: 10 (v/v), B: 甲醇, 0–14 min 10–50% B, 14–16 min 50–100% B, 16–18 min 100–10% B, 18–20 min 10% B, 0.4 mL/min |
| 檢測器 |
Waters QqTOF Premier 質譜儀 (Waters Corp., Manchester, UK) ESI 接口連接 UPLC 系統. 負離子模式, 毛細管: 2.1 kV, 錐電壓: 40 V, 源溫度: 120°C, 去溶劑溫度: 300°C, 錐: 0 L/h, 去溶劑: 500 L/h. |
|
|
|
| 儀器 |
Waters DP 4000 HPLC儀 |
| 色譜柱 |
反相 C18 色譜柱 (Symmetry prep™ C18, 7 μm, 19 mm × 300 mm), |
| 流動相 |
A: 甲醇-水 = 70: 30, v/v, B: 甲醇-水 = 30: 70 (1‰甲酸), v/v. 0-45 min 30% A, 45-80 min 30-60% A, 80-81 min 60% A, 81-120 min 60-30% A, 8 mL/min |
| 檢測器 |
UV λ288 nm, Waters 2487 dual λ 吸收光檢測器. |
|
|
|
| 儀器 |
Shimadzu LC-20AT HPLC儀 |
| 色譜柱 |
反相 C18 色譜柱 (250 mm × 4.6 mm, 5 µm, Diamodsil™), 30°C |
| 流動相 |
A: 甲醇, B: 水 (1% 甲酸), 0-3 min 43% A, 3-13 min 43-50% A, 13-25 min 50-60% A, 25-35 min 60% A, 35-35.01min 60-43% A, 35.01-45 min 43% A, 1 mL/min |
| 檢測器 |
UV λ288 nm |
|
|
|
| 儀器 |
Waters ACQUITY™ TQD 質譜及超高效液相色譜 (Waters, Milford, MA, USA) |
| 色譜柱 |
C18 色譜柱 (Sunfire™ C18, 2.1 mm × 50 mm, 3.5 μm, Waters, Milford, MA, USA), 30°C |
| 流動相 |
A: 乙腈, B: 水含0.3% 乙酸, 0-6.5 min 10-35% A, 0.2 mL/min |
| 檢測器 |
負離子模式, 去溶劑氣: 氮氣, 500 L/h, 源氣溫: 120°C, 去溶劑溫度: 350°C |
|
| 樣品製備 |
|
方法一 |
|
|
|
|
|
Model TBE-20 A HSCCC 儀 (Tauto Biotech, Shanghai, China) 包括三多層線圈 (總容量 20 mL, 2.6 mm I.D. PTFE 管). |
|
|
正己烷: 三氯甲烷: 甲醇: 水 = 0.5: 11: 10: 6 (0.5% 乙酸), v/v/v/v, 流動相: 下相 |
|
|
0.5 mL/min, 1700 rpm |
|
|
UV λ280 nm |
|
|
方法二 |
|
|
|
|
|
TBE-300A HSCCC儀 (Tauto Biotechnique, Shanghai, China) 包括三多層線圈 (i.d. = 1.5 mm, 總容量 = 260 mL)及20 mL樣品回路. |
|
|
正己烷: 三氯甲烷: 甲醇: 水 = 0.5: 11: 10: 6 (0.5% 乙酸), v/v/v/v, 流動相: 下相 |
|
|
2 mL/min, 850 rpm |
|
|
UV λ280 nm |
|
|
| 參考文獻 |
|
[1]
|
Gao, X., et al. (2013). "HPLC wavelength switching method for determination of different origin Polygonum orientale taxifolin and quercetin content." Zhongguo Xiandai Yingyong Yaoxue 30(5): 508-511. |
|
[2]
|
Zhang, Q.-F. and H.-Y. Cheung (2010). "The content of astilbin and taxifolin in concentrated extracts of Rhizoma Smilacis Glabrae and turtle jelly vary significantly." Food Chemistry 119(3): 907-912. |
|
[3]
|
Wang, Y.-H., et al. (2004). "Prevention of macrophage adhesion molecule-1 (Mac-1)-dependent neutrophil firm adhesion by taxifolin through impairment of protein kinase-dependent NADPH oxidase activation and antagonism of G protein-mediated calcium influx." Biochemical Pharmacology 67(12): 2251-2262. |
|
[4]
|
Wei, Y., et al. (2009). "Determination of taxifolin in Polygonum orientale and study on its antioxidant activity." Journal of Food Composition and Analysis 22(2): 154-157. |
|
[5]
|
Sun, X., et al. (2013). "Taxifolin prevents diabetic cardiomyopathy in vivo and in vitro by inhibition of oxidative stress and cell apoptosis." Food Chem Toxicol 63c: 221-232. |
|
[6]
|
Satue, M., et al. (2013). "Quercitrin and taxifolin stimulate osteoblast differentiation in MC3T3-E1 cells and inhibit osteoclastogenesis in RAW 264.7 cells." Biochem Pharmacol 86(10): 1476-1486. |
|
[7]
|
Casaschi, A., et al. (2004). "Inhibitory activity of diacylglycerol acyltransferase (DGAT) and microsomal triglyceride transfer protein (MTP) by the flavonoid, taxifolin, in HepG2 cells: potential role in the regulation of apolipoprotein B secretion." Atherosclerosis 176(2): 247-253. |
|
[8]
|
Wang, X.-d., et al. (2009). "Permeation of astilbin and taxifolin in Caco-2 cell and their effects on the P-gp." International Journal of Pharmaceutics 378(1–2): 1-8. |
|
[9]
|
Peng, M., et al. (2012). "The influence of Cd2+, Hg2+ and Pb2+ on taxifolin binding to bovine serum albumin by spectroscopic methods: With the viewpoint of toxic ions/drug interference." Environmental Toxicology and Pharmacology 33(2): 327-333. |
|
[10]
|
Vacek, J., et al. (2012). "Biotransformation of flavonols and taxifolin in hepatocyte in vitro systems as determined by liquid chromatography with various stationary phases and electrospray ionization-quadrupole time-of-flight mass spectrometry." Journal of Chromatography B 899(0): 109-115. |
|
[11]
|
Liu, H., et al. (2010). "Isolation and purification of silychristin, silydianin and taxifolin in the co-products of the silybin refined process from the silymarin by high-speed counter-current chromatography." Process Biochemistry 45(5): 799-804. |
|
[12]
|
Wang, X., et al. (2009). "A highly sensitive and robust UPLC–MS with electrospray ionization method for quantitation of taxifolin in rat plasma." Journal of Chromatography B 877(18–19): 1778-1786. |
|
| 連結 |
 中藥材圖像數據庫 中藥材圖像數據庫
 藥用植物圖像數據庫 藥用植物圖像數據庫
 中藥標本數據庫 中藥標本數據庫
|
 中藥材圖像數據庫
中藥材圖像數據庫
 藥用植物圖像數據庫
藥用植物圖像數據庫
 中藥標本數據庫
中藥標本數據庫


