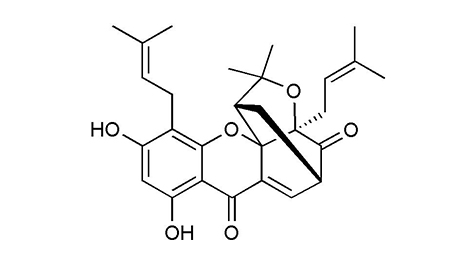
|
Natural Resources |
|
|
Bioactivities |
|
|
Identification |
|
| Analytical Method |
|
| INSTRUMENT |
Waters (Milford, MA, USA) HPLC system equipped with a 2695 Separations Module and a 2996 photodiode array detector |
| COLUMN |
SunFire C8 (2.1 × 150 mm, 3.5 μm) column (Waters Corporation, USA), 28°C |
| MOBILE PHASE |
Acetonitrile: methanol: 0.3% aqueous trifluoroacetic acid = 35.5: 33.5: 31 (v/v/v), 0.22 mL/min |
| DETECTION |
UV λ360 nm |
|
|
|
| INSTRUMENT |
Finnigan Surveyor LC Pump with an autosampler (Thermo Finnigan, San Jose, CA, USA) |
| COLUMN |
Luna C18 column (150 mm × 2.0 mm i.d., 3.0 μm, Phenomenex, Torrance, CA, USA) with a security C18 guard column (4 mm × 2.0 mm i.d., Phenomenex), 36°C |
| MOBILE PHASE |
Acetonitrile: 10 mM ammonium acetate in water = 75: 25, v/v, 0.26 mL/min |
| DETECTION |
Positive mode, spray: 4.0 kV, capillary: 320°C. Sheath gas: Nitrogen (30 Arb), ion Sweep Gas: Nitrogen (10 Arb), auxiliary gas: Nitrogen (10 Arb). The collision energy: 7 eV, collision-induced: dissociation (CID) and argon 1.0 mTorr, collision energy: 20 eV. |
|
|
|
| INSTRUMENT |
Waters ACQUITY UPLC™ system (Waters Corp., MS, USA), equipped with a binary solvent delivery system, autosampler, and a PDA detector |
| COLUMN |
Waters ACQUITY BEH C8 column (100 × 2.1 mm, 1.7 μm, Waters Corp., Ireland), 35°C |
| MOBILE PHASE |
A: 0.1% formic acid in water, B: acetonitrile containing 0.1% formic acid, 0-0.5 min 65% B, 0.5-1 min 65-75% B, 2-6 min 75% B, 6-7 min 75-95% B, 7-8 min 95% B, 0.3 mL/min |
| DETECTION |
Waters Q-TOF Premier (Micromass MS Technologies, Manchester, UK) operating in positive ion mode, nebulization gas: 650 L/h at 300°C, the cone gas: 50 L/h, the source: 90°C, capillary: 2700V, cone: 45 V |
|
| Sample Preparation |
|
METHOD 1 |
|
|
Fruit of sample was precolated with ethyl acetate and methanol, extract was fractionated by column chromatography. |
|
|
The ethyl acetate extract was separated by column chromatography (10 cm SiO2) using 1%, 2%, 3%, 5%, 10%, 15%, 20%, 25%, 30%, 40%, 60% of ethyl acetate-hexane and ethyl acetate and followed by 5%, 10%, 30%, 50%, 75% methanol-ethyl acetate and methanol. Collected and combined by TLC analysis to obtain fractions H1-H14. Fraction H9 was rechromatographed by column chromatography (Me2CO-hexane and methanol-Me2CO gradients, SiO2) to obtain fractions L1-L8. |
|
|
Fraction L5 (20-30% Me2CO-hexane) was separated by column chromatography (Me2CO-hexane and methanol-Me2CO gradients, SiO2) to give M1-M5 fractions. M3 gave targeted compound after recrystallization. |
|
|
| Reference |
|
[1]
|
Yang, J., et al. (2012). "Rapid characterization of caged xanthones in the resin of Garcinia hanburyi using multiple mass spectrometric scanning modes: The importance of biosynthetic knowledge based prediction." J. Pharm. Biomed. Anal. 60: 71-79. |
|
[2]
|
Hahnvajanawong, C., et al. (2010). "Apoptotic activity of caged xanthones from Garcinia hanburyi in cholangiocarcinoma cell lines." World J. Gastroenterol. 16(18): 2235-2243. |
|
[3]
|
Li, S.-L., et al. (2008). "Improved high-performance liquid chromatographic method for simultaneous determination of 12 cytotoxic caged xanthones in gamboges, a potential anticancer resin from Garcinia hanburyi." Biomed. Chromatogr. 22(6): 637-644. |
|
[4]
|
Theodorakis, E. and A. Batova (2008). Preparation of natural product xanthonoid small molecule analogs for therapeutic use in anticancer pharmaceutical compositions, The Regents of the University of California, USA. 52pp. |
|
[5]
|
Reutrakul, V., et al. (2007). "Cytotoxic and anti-HIV-1 caged xanthones from the resin and fruits of Garcinia hanburyi." Planta Med. 73(1): 33-40. |
|
[6]
|
Zhou, Y., et al. (2008). "Analysis of caged xanthones from the resin of Garcinia hanburyi using ultra-performance liquid chromatography/electrospray ionization quadrupole time-of-flight tandem mass spectrometry." Anal. Chim. Acta 629(1-2): 104-118. |
|