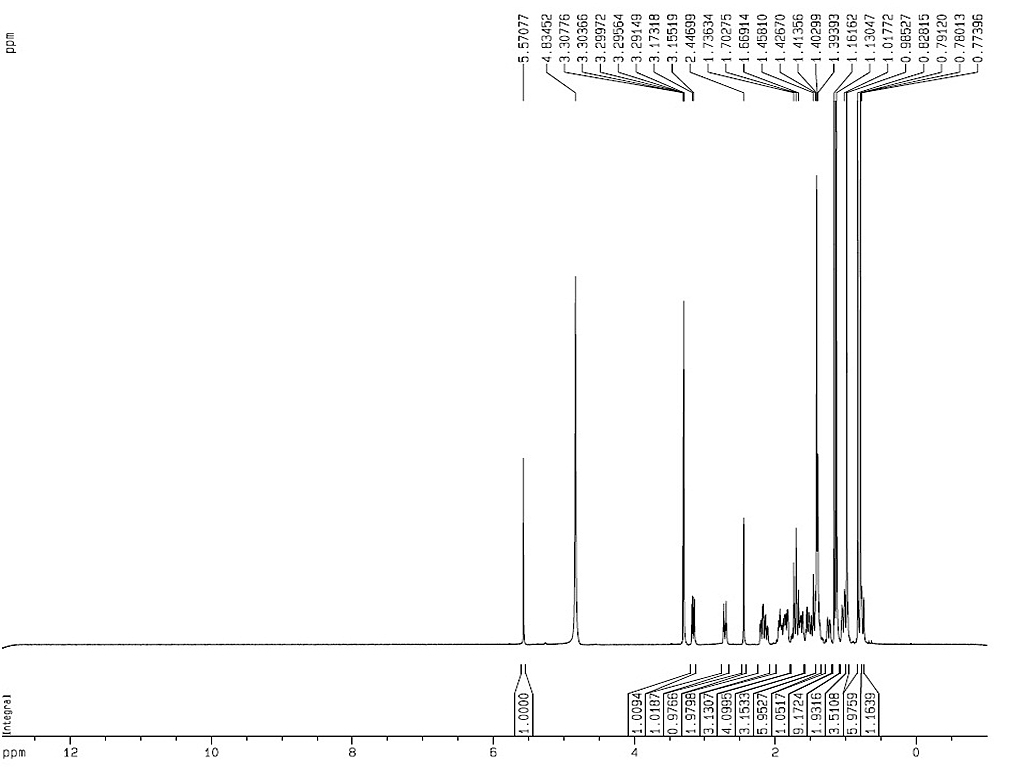
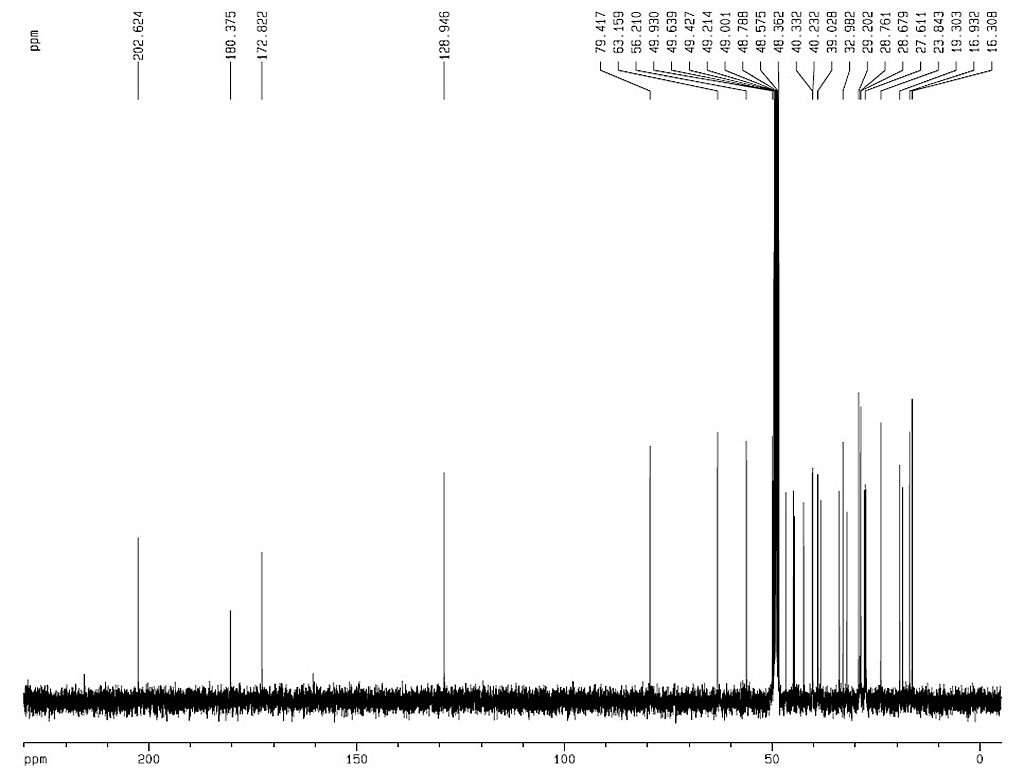
|
植物来源 |
|
|
生物活性 |
|
|
鉴定 |
熔点 |
300-304°C |
| 旋亮度 |
[α]D+161° (氯仿) |
|
|
| 分析方法 |
|
| 仪器 |
Merck TLC 铝板硅胶60 F254, Art. No. 5554 |
| 流动相 |
氯仿: 甲醇 (9: 1, v/v) |
| 检测器 |
UV λ254 nm |
|
|
|
| 仪器 |
Shimadzu LC-20AD series HPLC 仪 (Shimadzu, Kyoto, Japan) |
| 色谱柱 |
Zorbax XDB-C18 色谱柱 (2.1 mm × 50 mm i.d., 3.5 μm, Agilent Corporation, MA, USA) |
| 流动相 |
A: 0.1% 甲酸水, B: 0.1% 甲酸甲醇, 0.1-1.0 min 15-40% v/v B, 1.0-2.0 min 40-50% B, 2.0-2.3 min 50-90% B, 2.3-2.5 min 90-95% B, 2.5-4.5 min 95-98% B, 4.5-5.0 min 98% B, 5.01-7.00 min 15% B, 0.5 mL/min |
| 检测器 |
负离子模式; 多反映监视模式 (MRM); 离子源气1, 55 psi, 气2, 55 psi; 气帘, 20 psi; 离子喷雾电压, −4500 V; 500°C ; 氮气 |
|
|
|
| 仪器 |
ACQUITY UPLC™ 仪 (Waters Corp., Milford, MA, USA) |
| 色谱柱 |
ACQUITY UPLC™ BEH C18 色谱柱 (100 mm × 2.1 mm, 1.7 μm, 35°C) |
| 流动相 |
A: 甲醇, B: 水 = 2 mM 乙酸铵, 0-2.5 min 25-90% A, 2.5-4.5 min 90% A; 0.25 mL/min |
| 检测器 |
正离子模式; 毛细管电压 3.0 kV; 锥电压: 芍药苷13 kV, 柑橘黄苷15 kV, 柚配基30 kV, 甘草次酸20 kV, I.S. 30 kV; 源温度100°C, 去溶剂温度 450°C ; 氮气30 l/h, 锥气30 l/h. 氩气: 2.8 × 10−3 mbar. |
|
| 样品制备 |
|
方法一 |
|
|
微波辅助的加压的水解粗提 GA 21 min (15 min, 150°C, 保持 6 min) 150°C (450 W) 3-5% 硫酸溶液液固比 (ml/g crude GA) 25: U1. 获得产物达 90%. |
|
|
| 参考文献 |
|
[1]
|
Hayashi, H., et al. (1990). "Biotransformation of 18β-glycyrrhetinic acid by cell suspension cultures of Glycyrrhiza glabra." Phytochemistry 29(7): 2149-2152. |
|
[2]
|
Wang, Q.-e., et al. (2004). "Development of multi-stage countercurrent extraction technology for the extraction of glycyrrhizic acid (GA) from licorice (Glycyrrhiza uralensis Fisch)." Biochemical Engineering Journal 21(3): 285-292. |
|
[3]
|
Kalaiarasi, P., et al. (2009). "Hypolipidemic activity of 18β-glycyrrhetinic acid on streptozotocin-induced diabetic rats." European Journal of Pharmacology 612(1–3): 93-97. |
|
[4]
|
Kalaiarasi, P. and K. V. Pugalendi (2009). "Antihyperglycemic effect of 18β-glycyrrhetinic acid, aglycone of glycyrrhizin, on streptozotocin-diabetic rats." European Journal of Pharmacology 606(1–3): 269-273. |
|
[5]
|
Nafisi, S., et al. (2012). "Study on the interaction of glycyrrhizin and glycyrrhetinic acid with RNA." Journal of Photochemistry and Photobiology B: Biology 111(0): 27-34. |
|
[6]
|
Csuk, R., et al. (2011). "Synthesis and antitumor activity of ring A modified glycyrrhetinic acid derivatives." European Journal of Medicinal Chemistry 46(11): 5356-5369. |
|
[7]
|
Su, X., et al. (2007). "Inhibition of human and rat 11β-hydroxysteroid dehydrogenase type 1 by 18β-glycyrrhetinic acid derivatives." The Journal of Steroid Biochemistry and Molecular Biology 104(3–5): 312-320. |
|
[8]
|
Xu, C.-H., et al. "Pharmacokinetic comparisons of two different combinations of Shaoyao-Gancao Decoction in rats: Competing mechanisms between paeoniflorin and glycyrrhetinic acid." Journal of Ethnopharmacology (0). |
|
[9]
|
Wen, J., et al. (2012). "UPLC–MS/MS determination of paeoniflorin, naringin, naringenin and glycyrrhetinic acid in rat plasma and its application to a pharmacokinetic study after oral administration of SiNiSan decoction." Journal of Pharmaceutical and Biomedical Analysis 66(0): 271-277. |
|
[10]
|
Wang, R., et al. (2012). "Pressured Microwave-assisted Hydrolysis of Crude Glycyrrhizic Acid for Preparation of Glycyrrhetinic Acid." Chinese Journal of Chemical Engineering 20(1): 152-157. |
|
| 连结 |
 中药材图像数据库 中药材图像数据库
 药用植物图像数据库 药用植物图像数据库
 中药标本数据库 中药标本数据库
|
 中药材图像数据库
中药材图像数据库
 药用植物图像数据库
药用植物图像数据库
 中药标本数据库
中药标本数据库