|
Natural Resources |
|
|
Bioactivities |
|
|
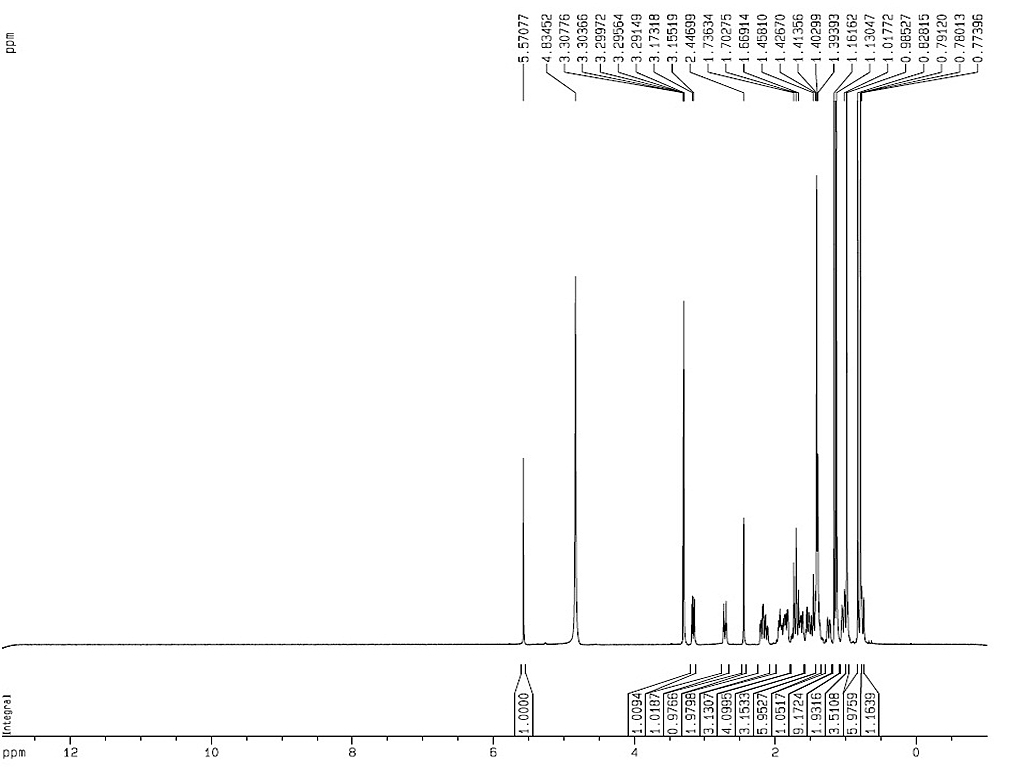
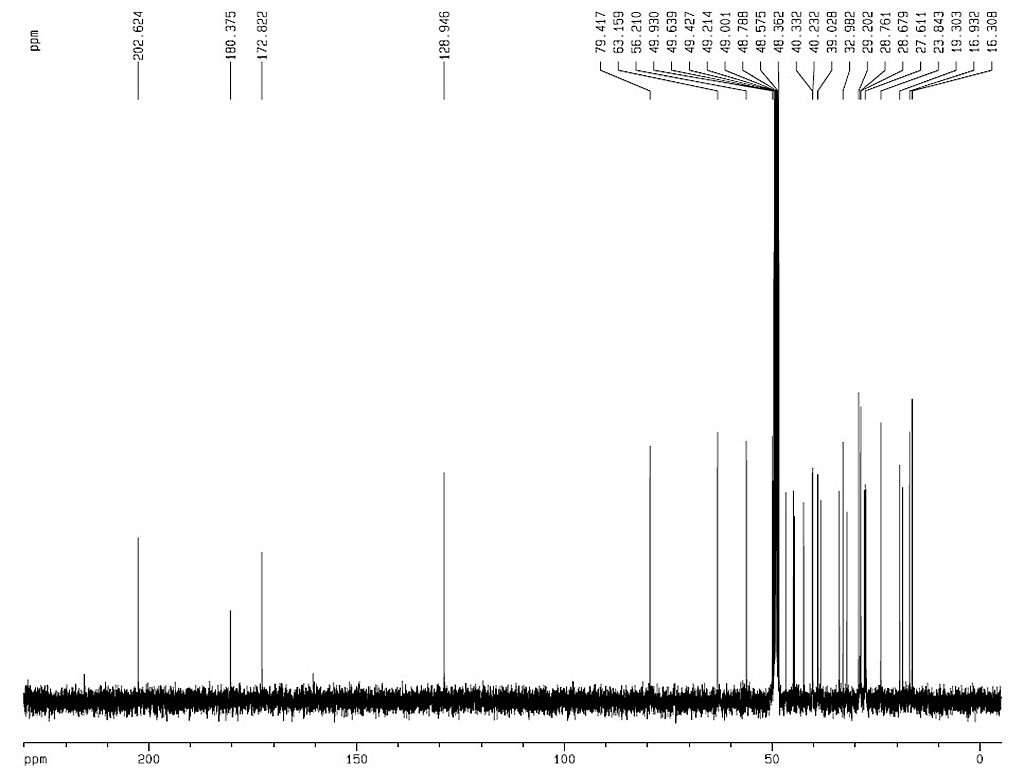
Identification |
Melting point |
300-304°C |
| Optical rotation |
[α]D+161° (CHCl3) |
|
|
| Analytical Method |
|
| INSTRUMENT |
Merck TLC aluminium sheets silica gel 60 F254, Art. No. 5554 |
| MOBILE PHASE |
Chloroform: Methanol (9: 1, v/v) |
| DETECTION |
UV λ254 nm |
|
|
|
| INSTRUMENT |
Shimadzu LC-20AD series HPLC system (Shimadzu, Kyoto, Japan) |
| COLUMN |
Zorbax XDB-C18 column (2.1 mm × 50 mm i.d., 3.5 μm, Agilent Corporation, MA, USA) |
| MOBILE PHASE |
A: 0.1% Formic acid in water, B: 0.1% Formic acid in methanol, 0.1-1.0 min 15-40% v/v B, 1.0-2.0 min 40-50% B, 2.0-2.3 min 50-90% B, 2.3-2.5 min 90-95% B, 2.5-4.5 min 95-98% B, 4.5-5.0 min 98% B, 5.01-7.00 min 15% B, 0.5 mL/min |
| DETECTION |
Negative mode; quantified by the multiple reaction monitoring (MRM) mode; Ion Source Gas1, 55 psi, Ion Source Gas2, 55 psi; Curtain Gas, 20 psi; Ion Spray Voltage, −4500 V; 500°C; Nitrogen |
|
|
|
| INSTRUMENT |
ACQUITY UPLC™ system (Waters Corp., Milford, MA, USA) |
| COLUMN |
ACQUITY UPLC™ BEH C18 column (100 mm × 2.1 mm, 1.7 μm, 35°C) |
| MOBILE PHASE |
A: methanol, B: water = 2 mM ammonium acetate, 0-2.5 min 25-90% A, 2.5-4.5 min 90% A; 0.25 mL/min |
| DETECTION |
Positive ionization mode; capillary voltage 3.0 kV; cone voltage: paeoniflorin 13 kV, naringin 15 kV, naringenin 30 kV, glycyrrhetinic acid 20 kVand I.S. 30 kV; source temperature 100°C, desolvation temperature 450°C ; Nitrogen 30 l/h, cone gas 30 l/h. Argon: 2.8 × 10-3 mbar. |
|
| Sample Preparation |
|
METHOD 1 |
|
|
Pressured microwave-assisted hydrolysis of crude GA for 21 min (taking 15 min to reach 150°C, and holding it for 6 min) at 150°C (at a radiation power of 450 W) in 3-5% sulfuric acid solution with the liquid-solid (ml/g crude GA) ratio of 25 : U1. The product yields up to 90%. |
|
|
| Reference |
|
[1]
|
Hayashi, H., et al. (1990). "Biotransformation of 18β-glycyrrhetinic acid by cell suspension cultures of Glycyrrhiza glabra." Phytochemistry 29(7): 2149-2152. |
|
[2]
|
Wang, Q.-e., et al. (2004). "Development of multi-stage countercurrent extraction technology for the extraction of glycyrrhizic acid (GA) from licorice (Glycyrrhiza uralensis Fisch)." Biochemical Engineering Journal 21(3): 285-292. |
|
[3]
|
Kalaiarasi, P., et al. (2009). "Hypolipidemic activity of 18β-glycyrrhetinic acid on streptozotocin-induced diabetic rats." European Journal of Pharmacology 612(1–3): 93-97. |
|
[4]
|
Kalaiarasi, P. and K. V. Pugalendi (2009). "Antihyperglycemic effect of 18β-glycyrrhetinic acid, aglycone of glycyrrhizin, on streptozotocin-diabetic rats." European Journal of Pharmacology 606(1–3): 269-273. |
|
[5]
|
Nafisi, S., et al. (2012). "Study on the interaction of glycyrrhizin and glycyrrhetinic acid with RNA." Journal of Photochemistry and Photobiology B: Biology 111(0): 27-34. |
|
[6]
|
Csuk, R., et al. (2011). "Synthesis and antitumor activity of ring A modified glycyrrhetinic acid derivatives." European Journal of Medicinal Chemistry 46(11): 5356-5369. |
|
[7]
|
Su, X., et al. (2007). "Inhibition of human and rat 11β-hydroxysteroid dehydrogenase type 1 by 18β-glycyrrhetinic acid derivatives." The Journal of Steroid Biochemistry and Molecular Biology 104(3–5): 312-320. |
|
[8]
|
Xu, C.-H., et al. "Pharmacokinetic comparisons of two different combinations of Shaoyao-Gancao Decoction in rats: Competing mechanisms between paeoniflorin and glycyrrhetinic acid." Journal of Ethnopharmacology (0). |
|
[9]
|
Wen, J., et al. (2012). "UPLC–MS/MS determination of paeoniflorin, naringin, naringenin and glycyrrhetinic acid in rat plasma and its application to a pharmacokinetic study after oral administration of SiNiSan decoction." Journal of Pharmaceutical and Biomedical Analysis 66(0): 271-277. |
|
[10]
|
Wang, R., et al. (2012). "Pressured Microwave-assisted Hydrolysis of Crude Glycyrrhizic Acid for Preparation of Glycyrrhetinic Acid." Chinese Journal of Chemical Engineering 20(1): 152-157. |
|
| Link to |
 Chinese Medicinal Material Images Database Chinese Medicinal Material Images Database
 Medicinal Plant Images Database Medicinal Plant Images Database
 Chinese Medicine Specimen Database Chinese Medicine Specimen Database
|
 Chinese Medicinal Material Images Database
Chinese Medicinal Material Images Database
 Medicinal Plant Images Database
Medicinal Plant Images Database
 Chinese Medicine Specimen Database
Chinese Medicine Specimen Database