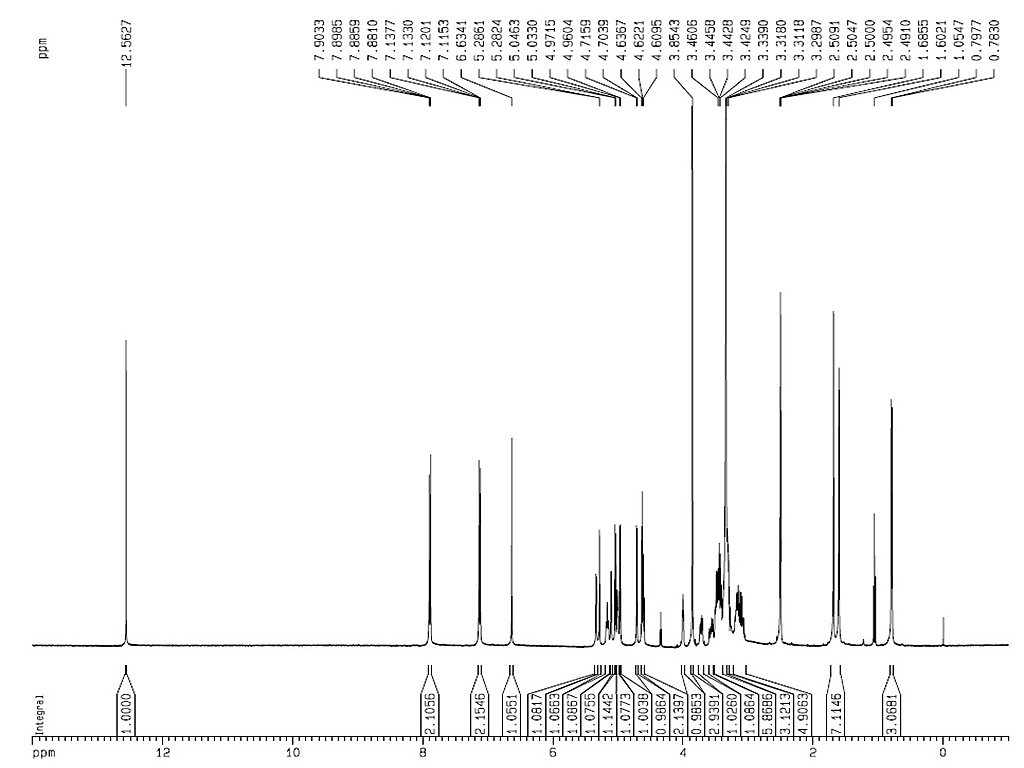
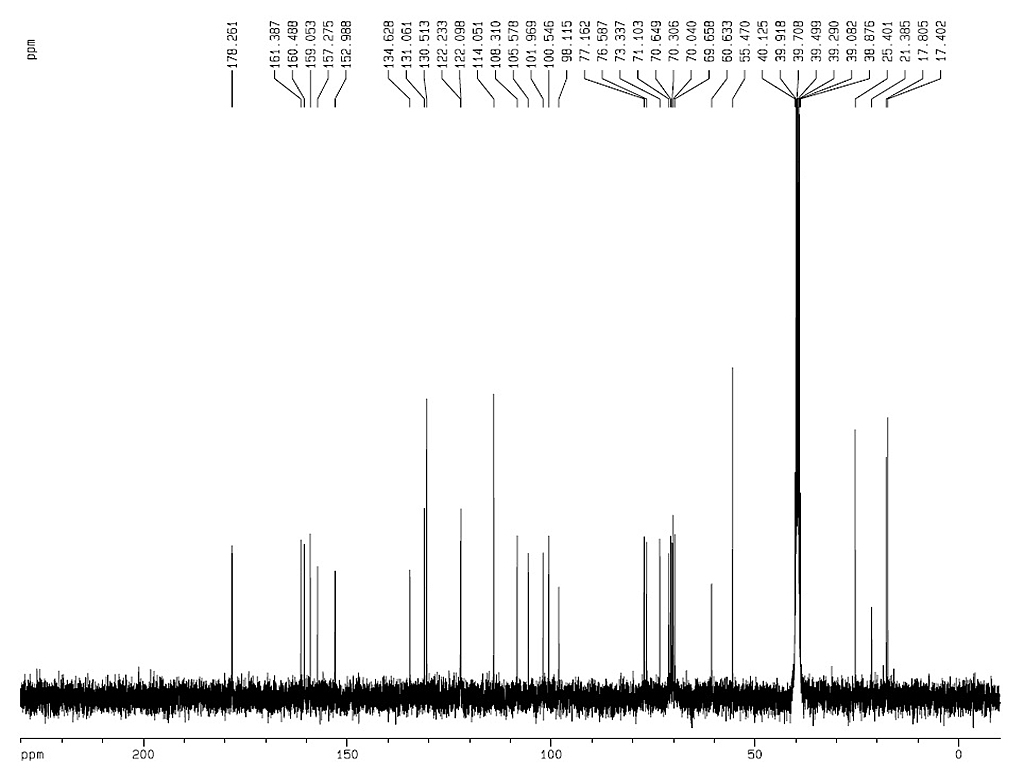
|
Natural Resources |
|
|
Bioactivities |
|
|
Identification |
Melting point |
231-235°C (226-229°C) |
| Optical rotation |
[α]15D-87.1° (Py) |
|
|
| Analytical Method |
|
| INSTRUMENT |
Silica gel H plate |
| MOBILE PHASE |
Chloroform: methanol: formic acid = 15: 1: 0.2, v/v |
| DETECTION |
Visualized by spraying 5% Ferric Chloride in Ethanol |
|
|
|
| INSTRUMENT |
LC-10ATVP HPLC pump, Shimadzu, Japan |
| COLUMN |
ODS column (Shim-pack VP-ODS 4.6 mm × 150 mm, 5.0 μm, Dikma, Beijing) |
| MOBILE PHASE |
Acetonitrile: 3% acetic acid = 29: 71, v: v, 1.0, 1.1 and 1.0 mL/min |
| DETECTION |
UV λ270 nm |
|
|
|
| INSTRUMENT |
Waters Acquity™ with diode array detector (DAD) |
| COLUMN |
Acquity UPLC BEH C18 column (50 mm × 2.1 mm i.d., 1.7 mm; Waters, Milford, MA, USA) |
| MOBILE PHASE |
A: 100% aqueous buffer = 2.5 mM NH4Ac, pH 7.4, B: 100% acetonitrile, 0.45 mL/min |
| DETECTION |
UV λ254 nm |
|
|
|
| INSTRUMENT |
The Agilent G6410A triple quadrupole LC-MS system |
| COLUMN |
ZORBAX SB-C18 column (3.5 μm, 2.1 mm × 100 mm) and a C18 guard column (5 μm, 4.0 mm × 2.0 mm), 35°C |
| MOBILE PHASE |
Acetonitrile: water: Formic acid = 50: 50: 0.05, v/v/v, 0.25 mL/min |
| DETECTION |
Positive mode, 4000 V, nebulizer gas 40 psi, 10 L/min |
|
| Sample Preparation |
|
METHOD 1 |
|
|
Two-phase solvent system composed of n-hexane-n-butanol-methanol-water (1: 4: 2: 6, v/v). Two multilayer coil planet centrifuges with different capacities. A 300 mg amount of the extract was separated using a semipreparative instrument equipped with a 230-ml capacity column to yield 103 mg of icariin at 86.2% purity. |
|
|
An 8 g amount of the extract was separated with a large preparative instrument equipped with a 2460-ml capacity column to produce 2.45 g of icariin at 85.7% purity. From these fractions over 98% pure icariin was obtained by recrystallization with water. |
|
|
| Reference |
|
[1]
|
Yang, L., et al. (2013). "Icariin from Epimedium brevicornum Maxim promotes the biosynthesis of estrogen by aromatase (CYP19)." Journal of Ethnopharmacology 145(3): 715-721. |
|
[2]
|
Hsieh, T.-P., et al. (2010). "Icariin isolated from Epimedium pubescens regulates osteoblasts anabolism through BMP-2, SMAD4, and Cbfa1 expression." Phytomedicine 17(6): 414-423. |
|
[3]
|
Li, H.-f., et al. (2012). "Antioxidant flavonoids from Epimedium wushanense." Fitoterapia 83(1): 44-48. |
|
[4]
|
Zhang, D. W., et al. (2008). "Effects of total flavonoids and flavonol glycosides from Epimedium koreanum Nakai on the proliferation and differentiation of primary osteoblasts." Phytomedicine 15(1–2): 55-61. |
|
[5]
|
Wang, L., et al. (2009). "Icariin enhances neuronal survival after oxygen and glucose deprivation by increasing SIRT1." European Journal of Pharmacology 609(1–3): 40-44. |
|
[6]
|
Wang, F., et al. (2012). "Icariin enhances the healing of rapid palatal expansion induced root resorption in rats." Phytomedicine 19(11): 1035-1041. |
|
[7]
|
Zhu, J., et al. (2008). "TLC identification for four medicinal materials and the determination of icariin in Tianshenyizhi capsule." Shizhen Guoyi Guoyao 19(2): 324-326. |
|
[8]
|
Li, Y., et al. (2009). "In vivo pharmacokinetics comparisons of icariin, emodin and psoralen from Gan-kang granules and extracts of Herba Epimedii, Nepal dock root, Ficus hirta yahl." Journal of Ethnopharmacology 124(3): 522-529. |
|
[9]
|
Liu, W., et al. (2011). "Sensitive and robust UPLC–MS/MS method to determine the gender-dependent pharmacokinetics in rats of emodin and its glucuronide." Journal of Pharmaceutical and Biomedical Analysis 54(5): 1157-1162. |
|
[10]
|
Xu, W., et al. (2007). "LC–MS/MS method for the simultaneous determination of icariin and its major metabolites in rat plasma." Journal of Pharmaceutical and Biomedical Analysis 45(4): 667-672. |
|
[11]
|
Du, Q., et al. (2002). "Purification of icariin from the extract of Epimedium segittatum using high-speed counter-current chromatography." Journal of Chromatography A 962(1–2): 239-241. |
|
| Link to |
 Chinese Medicinal Material Images Database Chinese Medicinal Material Images Database
 Medicinal Plant Images Database Medicinal Plant Images Database
 Chinese Medicine Specimen Database Chinese Medicine Specimen Database
|
 Chinese Medicinal Material Images Database
Chinese Medicinal Material Images Database
 Medicinal Plant Images Database
Medicinal Plant Images Database
 Chinese Medicine Specimen Database
Chinese Medicine Specimen Database