|
Natural Resources |
|
|
Synonyms |
|
|
Bioactivities |
|
|
Identification |
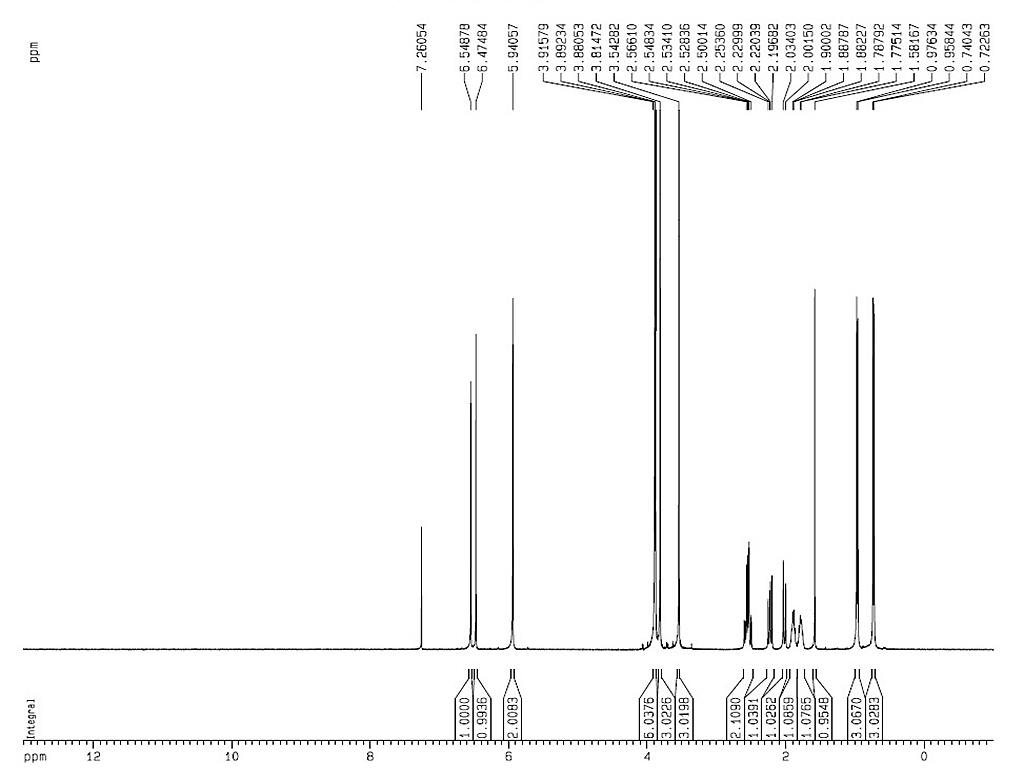
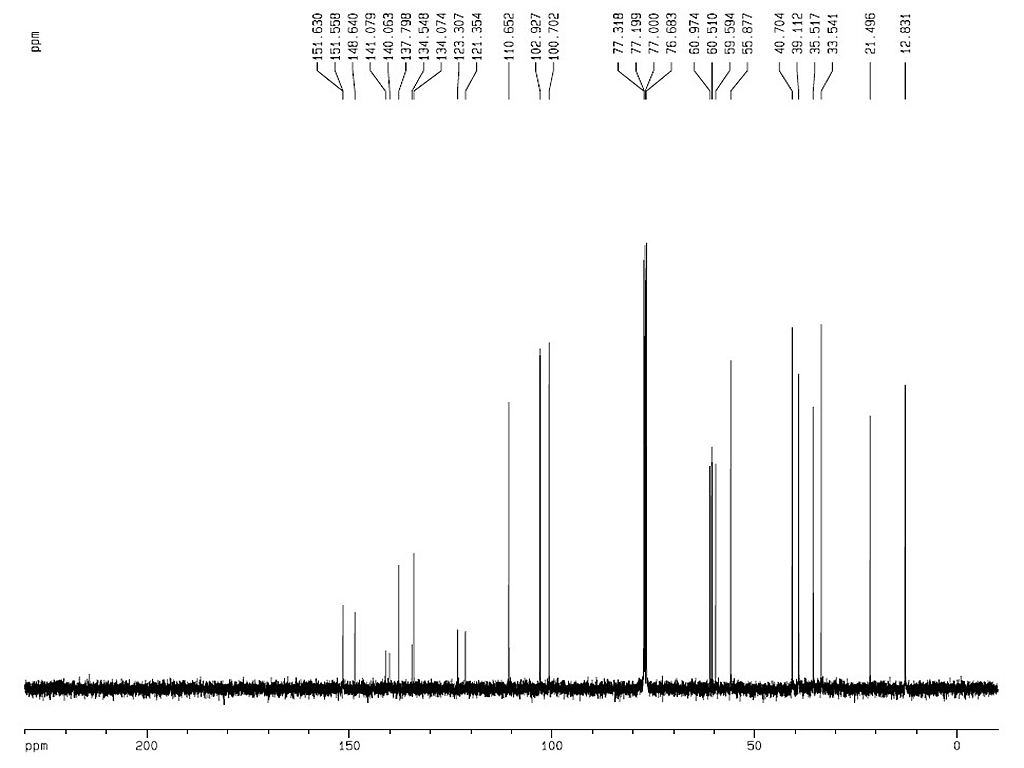
Melting point |
117-119°C |
| Optical rotation |
[α]D28±3° (C=3.0, chloroform) |
|
|
| Analytical Method |
|
| INSTRUMENT |
High performance silica gel GF254 plate |
| MOBILE PHASE |
Petroleum ether: ethyl formate: formic acid = 15: 5: 1 |
| DETECTION |
UV λ254 nm |
|
|
|
| INSTRUMENT |
Shimadzu LC-2010 (Shimadzu Corp., Kyoto, Japan) and Shimadzu LC solution software. |
| COLUMN |
Shimadzu C18 column (Phenomenex, 150 × 4.6 mm, 5μm particle size). |
| MOBILE PHASE |
Methanol: deionized water = 70: 30, 0.8 mL/min |
| DETECTION |
UV λ280 nm |
|
|
|
| INSTRUMENT |
Agilent 1100 HPLC (Agilent Technologies, Palo Alto, CA, USA), equipped with a quaternary pump, an auto-sampler, a vacuum degasser, an automatic thermostatic column compartment and a UV detector. |
| COLUMN |
Elite ODS C18 column (250 mm × 4.6 mm, 5 μm) |
| MOBILE PHASE |
A: acetonitrile, B: water, 1.0 mL/min |
| DETECTION |
UV λ217 nm |
|
|
|
| INSTRUMENT |
Q TRAPTM 4000 MS/MS system from Applied AB Sciex (Foster City, CA, USA) coupled to a Prominence™ UFLC system (Shimadzu, Kyoto, Japan) |
| COLUMN |
Shim-pack XR-ODS column (75 mm × 3.0 mm, 2.2 μm) (Shimadzu, Kyoto, Japan) |
| MOBILE PHASE |
A: 0.1% formic acid water, B: acetonitrile, 0.4 mL/min |
|
| Sample Preparation |
|
METHOD 1 |
|
|
|
|
|
Model TBE-300, Shanghai Tauto Biological Company, China) was equipped with three preparative coils connected in series (diameter of polytetrafluoroethylene (PTFE) tube, 2.6 mm; total volume, 119 ml) and a 10ml sample loop. |
|
|
n-hexane: methanol: water = 35: 30: 3, v/v |
|
|
1.0 mL/min |
|
|
UV λ254 nm |
|
|
| Reference |
|
[1]
|
Guo, L. Y., et al. (2008). "Anti-inflammatory effects of schisandrin isolated from the fruit of Schisandra chinensis Baill." European Journal of Pharmacology 591(1–3): 293-299. |
|
[2]
|
Liu, H., et al. (2012). "Chemical analysis of twelve lignans in the fruit of Schisandra sphenanthera by HPLC–PAD-MS." Phytomedicine 19(13): 1234-1241. |
|
[3]
|
Chiu, P. Y., et al. (2008). "Schisandrin B stereoisomers protect against hypoxia/reoxygenation-induced apoptosis and inhibit associated changes in Ca2+-induced mitochondrial permeability transition and mitochondrial membrane potential in H9c2 cardiomyocytes." Life Sciences 82(21–22): 1092-1101. |
|
[4]
|
Lam, P. Y., et al. (2011). "Schisandrin B protects against solar irradiation-induced oxidative stress in rat skin tissue." Fitoterapia 82(3): 393-400. |
|
[5]
|
Ip, S.-P. and K.-M. Ko (1996). "The crucial antioxidant action of schisandrin B in protecting against carbon tetrachloride hepatotoxicity in mice: A comparative study with butylated hydroxytoluene." Biochemical Pharmacology 52(11): 1687-1693. |
|
[6]
|
LI Li-shun, S. W.-j., WANG Gui-yang (2011). "Identification and Determination of Three Kinds of Schisandra by HPTLC and HPLC." 《Lishizhen Medicine and Materia Medica Research》 22(1008-0805). |
|
[7]
|
Gu, W., Wei, N., & Wang, Z. (2008). "LC analysis of lignans from Schisandra sphenanthera Rehd. et Wils. Chromatographia", 67(11/12), 979-983. doi: 10.1365/s10337-008-0621-7 |
|
[8]
|
Lu, T.-L., et al. (2012). "Quality analysis of raw and processed Schisandra chinensis fructus by simultaneous determination of eleven bioactive lignans using RP-HPLC method." J. Food Drug Anal. 20(4): 922-929. |
|
[9]
|
Wei, B., et al. (2013). "Development of a UFLC–MS/MS method for simultaneous determination of six lignans of Schisandra chinensis (Turcz.) Baill. in rat plasma and its application to a comparative pharmacokinetic study in normal and insomnic rats." Journal of Pharmaceutical and Biomedical Analysis 77(0): 120-127. |
|
[10]
|
Huang, T., et al. (2005). "Preparative separation and purification of deoxyschisandrin and γ-schisandrin from Schisandra chinensis (Turcz.) Baill by high-speed counter-current chromatography." Journal of Chromatography A 1066(1–2): 239-242. |
|
| Link to |
 Chinese Medicinal Material Images Database Chinese Medicinal Material Images Database
 Medicinal Plant Images Database Medicinal Plant Images Database
 Chinese Medicine Specimen Database Chinese Medicine Specimen Database
|
 Chinese Medicinal Material Images Database
Chinese Medicinal Material Images Database
 Medicinal Plant Images Database
Medicinal Plant Images Database
 Chinese Medicine Specimen Database
Chinese Medicine Specimen Database