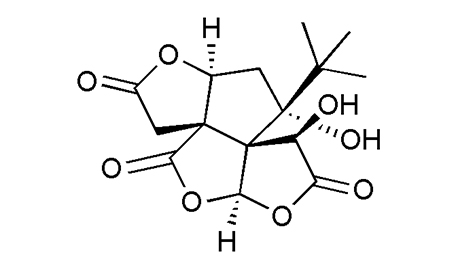
|
植物來源 |
|
|
生物活性 |
|
|
鑑定 |
熔點 |
300°C |
| 旋光度 |
[α]20D-66.6° |
1HNMR


|
| 分析方法 |
|
| 儀器 |
矽膠F254 板及乙酸鈉 |
| 流動相 |
乙酸乙酯: 己烷 (9: 1) (不飽和) 或100% 乙酸甲酯 (飽和) |
|
|
|
| 儀器 |
Agilent 1100 高校液相色譜儀 (Agilent Corporation, USA); 2000ES 蒸發光散射品質檢測器 (ELSD) (Alltech Corporation) |
| 色譜柱 |
ODS-C 18柱 (4.6 mm × 250 mm, 5 µm) (Dalian Elite Analytical Instruments Co., Ltd.) |
| 流動相 |
甲醇-四氫呋喃-水 (V/V/V, 25: 5: 70) 恒組成溶劑洗脫系統, 1.0 mL/min |
|
|
|
| 儀器 |
Series 1100 儀 (Agilent Technologies, Palo Alto, CA) |
| 色譜柱 |
Gemini octadecylsilyl (C18) 色譜柱 (150 × 4.6 mm id, 5 µm; Phenomenex, Torrance, CA). 配備一個保護柱 (4 × 2.0 mm, Part No. KJO-4282, Phenomenex) |
| 流動相 |
A: 0.1% 甲酸水, B: 0.1% 甲酸乙腈, 0-20 min, 15-100% B, 0.5 mL/min |
|
|
|
| 儀器 |
ACQUITY UPLC™ 儀 (Waters Corp., Milford, MA), ACQUITY UPLC Shield RP18 色譜柱 (50 × 2.1 mm), 1.7 µm 球形多孔顆粒 |
| 色譜柱 |
ACQUITY 色譜柱配備 LC-18 保護柱 (Vanguard 2.1 × 5 mm, Waters Corp.) |
| 流動相 |
0.05% 甲酸水 (A), 0.05% 甲酸乙腈 (B), 0-7 min, 90-75% A, 0.3 mL/min |
|
|
|
| 儀器 |
ThermoFinnigan 氣象色譜儀 (Rodano, Italy), 配備FID 檢測器 (3 pg C/s, 線性10 6) 及兩個注射進樣器: 分流/不分流及冷柱頭進樣. 使用分流模式. |
| 色譜柱 |
19091J-413, 30 m x 0.25mm i.d., 固定相HP-5, 膜厚度0.25 µ m (J&W Scientific, Folsom, USA), Hamilton model no. 701注射器 (Bonaduz, Switzerland). 溫度300°C, 分流比1: 10, 分流流率17 mL/min. 程序設計柱箱溫度50°C (2 min) 至300°C (for 9.7 min)升溫速率30°C min-1.FID基溫度: 325°C |
| 流動相 |
載氣氦氣 5.0 純級 (Linde Gaz, Poland). 過濾器 OT3-2, R&D 氧氣乾燥器, R&D 分離. 記錄載氣流率 1.7 mL/min (35 cm/s). 流入ñ合成氣 (350 mL/min), 氫氣 (35 mL/min) 和氮氣 (補充氣, 33 mL/min) 5.0純級 (Linde Gaz, Poland) |
|
| 樣品製備 |
|
方法一 |
|
|
乙酸乙酯 (5-10 mL) 提取的樣品 (4.0 g) 上樣矽膠柱 (100 g). 乙酸乙酯/己烷洗脫. 初始容積系統 EtOAc/hexanes (3.5: 6.5, 500 mL). EtOAc 分六個梯度增加 (4: 6, 500 mL; 4.5: 5.5, 500 mL; 5: 5, 500 mL; 5.5: 4.5, 500 mL; 6: 4, 400 mL; 6.5: 3.5, 100 mL). 收集: EtOAc/hexanes (45: 55): 銀杏內酯 (0.4 g). (50: 50): 粗提 BB (1) 和 GA (2) 合併 GA/GB. (55: 45): GA/GB (1.1 g). (40: 60): GC/GJ (0.4 g) 包含 GA (2) 和 GB (3). Et2O 乙醚部內銀杏內酯 (2 mL), Et2O (1 mL) 過濾沖洗得純銀杏內酯 (217 mg 1HnmR P 98%). |
|
|
銀杏提取物 (25 g) 或銀杏葉 (250 g) 煎煮 1 小時, 得 0.5 L (提取物) 或 2 L (銀杏葉) 5% H2O2 溶液 (或其他氧化劑水溶液). EtOAc (250/125/75 mL) 提取三次. 水、80% NaCl水溶液 (稀釋至 80%)、飽和鹽溶液 (NaHCO3/ Na2S2O3, NaHCO3/ Na2S2O3, or Na2SO3) 沖洗有機層. 乾燥後加入 50-60% 萜烯內酯. Dianon HP-20 30 或 Amberlite XAD-16 高分子樹脂應用反相色譜以甲醇水溶液分離 (30-90%). 合併萜烯內酯餾分, 去除溶劑. 60% 甲醇溶液填充 WP C18 色譜柱兩次洗脫去除銀杏酸. |
|
|
己烷提取物 G 中分離標準品.上樣矽膠色譜柱 (1% 甲醇氯仿), RP-18 矽膠柱 (0-100% 甲醇, 保留 100% 甲醇餾分), 半製備HPLC製備 (1 × 25 cm 5 µm YMC ODS-AM 色譜柱, H2O/MeOH/AcOH (100: 10: 1), 215 µm 檢測波長) |
|
|
| 參考文獻 |
|
[1]
|
Chang, T. K. H., et al. (2006). "Effect of Ginkgo biloba extract on procarcinogen-bioactivating human CYP1 enzymes: Identification of isorhamnetin, kaempferol, and quercetin as potent inhibitors of CYP1B1." Toxicology and Applied Pharmacology 213(1): 18-26. |
|
[2]
|
Bouaziz, N., et al. (2002). "Mitochondrial respiratory chain as a new target for anti-ischemic molecules." European Journal of Pharmacology 441(1-2): 35-45. |
|
[3]
|
Qin, H., Song, F., Han, H., Qu, H., Zhai, X., Qin, B., et al. (2011). "Bilobalide inhibits the expression of aquaporin 1, 4 and glial fibrillary acidic protein in rat brain tissue after permanent focal cerebral ischemia." Neural Regeneration Research, 2105-2111. |
|
[4]
|
Teris A. van Beek*, Gerrit P. Lelyveld, "Thin layer chromatography of bilobalide and ginkgolides A, B, C and J on sodium acetate impregnated silica gel." Phytochemical Analysis Volume 4, Issue 3, pages 109-114, May or June 1993 |
|
[5]
|
WANG Xin, L. J. (2012). "Study on Ginkgolides of Wild Ginkgo boliba L. Leaves from Three Gorges Reservoir Areas of Chongqing City." Medicinal Plant, p. 17-19. |
|
[6]
|
Leung, A.Y., & Foster, S. (1996) "Encyclopedia of Common Natural Ingredients Used in Food." Drugs, and Cosmetics, John Wiley & Sons, New York, NY |
|
[7]
|
Bharthi Avula, Yan-Hong Wang, and Troy J. Smille. (2009). "Column Liquid Chromatography/Electrospray Ionization-Time of Flight-Mass Spectrometry and Ultraperformance Column Liquid Chromatography Mass Spectrometry Methods for the Determination of Ginkgolides and Bilobalide in the Leaves of Ginkgo biloba and Dietary Supplements." Journal of AOAC International, pp. 645-652. |
|
[8]
|
Jan Krzek, Janusz S. Czekaj, Wlodzimierz Rzeszutoko and Radoslaw J. Ekiert. (2007)."Validation of Capillary Gas Chromatographic Method for Determination of Bilobalide and Ginkgolides A, B, C in Ginkgo Biloba Dry and Liquid Extracts." Polish Pharmaceutical Society, pp. 303-310 |
|
[9]
|
Stanislav Jaracz, S. M. (2004). "Isolation of ginkgolides A, B, C, J and bilobalide from G. biloba extracts." Phytochemistry, pp. 2897-2902. |
|
[10]
|
Dirk Lichtblau, J. M. (2002). "Efficient Extraction of Ginkgolides and Bilobalide from Ginkgo biloba Leaves." J. Nat. Prod., pp. 1501 - 1504. |
|