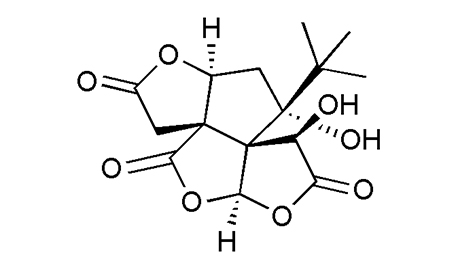
|
Natural Resources |
|
|
Bioactivities |
|
|
Identification |
Melting point |
300°C |
| Optical rotation |
[α]20D-66.6° |
1HNMR


|
| Analytical Method |
|
| INSTRUMENT |
Silica gel F254 plates with sodium acetate |
| MOBILE PHASE |
Ethyl acetate: hexane (9: 1) (non-saturated chamber) or 100% methyl acetate (saturated chamber) |
|
|
|
| INSTRUMENT |
Agilent 1100 high performance liquid chromatograph (Agilent Corporation, USA); 2000ES evaporative light-scatting mass detector (ELSD) (Alltech Corporation) |
| COLUMN |
ODS-C 18 (4.6 mm × 250 mm, 5 µm) (Dalian Elite Analytical Instruments Co., Ltd.) |
| MOBILE PHASE |
An isocratic elution system of methanol-tetrahydrofuran-water (V/V/V, 25: 5: 70), 1.0 mL/min |
|
|
|
| INSTRUMENT |
Series 1100 (Agilent Technologies, Palo Alto, CA) |
| COLUMN |
Separation was achieved on a Gemini octadecylsilyl (C18) column (150 × 4.6 mm id, 5 µm particle size; Phenomenex, Torrance, CA). The column was equipped with a security guard column (4 × 2.0 mm, Part No. KJO-4282, Phenomenex) |
| MOBILE PHASE |
A: Water with 0.1% formic acid, B: acetonitrile with 0.1% formic acid, 0-20 min, 15-100% B, 0.5 mL/min |
|
|
|
| INSTRUMENT |
ACQUITY UPLC™ system (Waters Corp., Milford, MA) using an RP column ACQUITY UPLC Shield RP18 (50 × 2.1 mm) with 1.7 µm spherical porous particles |
| COLUMN |
ACQUITY column was equipped with an LC-18 guard column (Vanguard 2.1 × 5 mm, Waters Corp.) |
| MOBILE PHASE |
Water with 0.05% formic acid (A) and acetonitrile with 0.05% formic acid (B), 0-7 min, 90-75% A, 0.3 mL/min |
|
|
|
| INSTRUMENT |
ThermoFinnigan (Rodano, Italy), equipped with a FID detector (3 pg C/s, linearity 10 6) and two injectors: split-splitless and cool on-column has been used. The split mode of split-splitless port was used. |
| COLUMN |
19091J-413, 30 m x 0.25mm i.d., with the stationary phase HP-5, film thickness 0.25 µ m (J&W Scientific, Folsom, USA), Hamilton syringe model no. 701 (Bonaduz, Switzerland). In temperature of 300°C, and split ratio 1: 10, the split flow equals 17 mL/min. The temperature in oven was programmed from 50°C (for 2 min) up to300°C (for 9.7 min) and the rate was 30°C min-1. FID base body temperature: 325°C |
| MOBILE PHASE |
As carrier gas, helium of purity class 5.0 (Linde Gaz, Poland) was used. It additionally passed through the filter OT3-2, R&D Oxygen/Moisture Trap, R&D Separations. The chromatograms were recorded at constant carrier gas flow of 1.7 mL/min (35 cm/s). The following gases were delivered to the detector ñ synthetic air (350 mL/min), hydrogen (35 mL/min) and nitrogen (make-up gas, 33 mL/min) of purity class 5.0 (Linde Gaz, Poland) |
|
| Sample Preparation |
|
METHOD 1 |
|
|
Extract (4.0 g) in EtOAc (5-10 mL) was loaded on a silica gel (100 g) column. Eluted with EtOAc/hexanes. The initial solvent system was EtOAc/hexanes (3.5: 6.5, 500 mL). EtOAc was increased in six steps (4: 6, 500 mL; 4.5: 5.5, 500 mL; 5: 5, 500 mL; 5.5: 4.5, 500 mL; 6: 4, 400 mL; 6.5: 3.5, 100 mL). Collection: EtOAc/hexanes (45: 55): bilobalide (0.4 g). (50: 50): impure BB (1) and GA (2) then mixture GA/GB. (55: 45): mixture GA/GB (1.1 g). (40: 60): mixture of GC/GJ (0.4 g) with small amount of GA (2) and GB (3). Bilobalide suspended in Et2O (2 mL) diethyl ether, filtered and washed twice with Et2O (1 mL) to yield pure bilobalide (217 mg purity by 1HnmR P 98%). |
|
|
Bioginkgo extract (25 g) or leaves (250 g) were boiled for 1 hour in 0.5 L (extract) or 2 L (leaves) of 5% aqueous H2O2 (or other oxidation reagents in water). Extracted three times with EtOAc (250/125/75 mL). Saturated aqueous salt solution (NaHCO3/ Na2S2O3, NaHCO3/ Na2S2O3, or Na2SO3) followed by water and 80% aqueous NaCl (saturated aqueous solution diluted to 80%) to wash the organic layer. Added 50 - 60% terpene trilactone content after drying. Reversed-phased chromatography using polymeric resins such as Dianon HP-20 30 or Amberlite XAD-16 by increasing methanol (30-90%) in water. Combined terpene trilactone-containing fractions, and removed solvent. WP C18 silica twice with aqueous 60% methanol to remove ginkgolic acids. |
|
|
Standards were isolated from hexane-extracted G. biloba leaves that were subjected to chromatography with silica gel (1% MeOH in CHCl3), RP-18 silica gel (0-100% MeOH, retaining the 100% MeOH fraction), and semipreparative HPLC (1 × 25 cm 5 µm YMC ODS-AM column, H2O/MeOH/AcOH (100: 10: 1) solvent system, monitored at 215 µm) |
|
|
| Reference |
|
[1]
|
Chang, T. K. H., et al. (2006). "Effect of Ginkgo biloba extract on procarcinogen-bioactivating human CYP1 enzymes: Identification of isorhamnetin, kaempferol, and quercetin as potent inhibitors of CYP1B1." Toxicology and Applied Pharmacology 213(1): 18-26. |
|
[2]
|
Bouaziz, N., et al. (2002). "Mitochondrial respiratory chain as a new target for anti-ischemic molecules." European Journal of Pharmacology 441(1-2): 35-45. |
|
[3]
|
Qin, H., Song, F., Han, H., Qu, H., Zhai, X., Qin, B., et al. (2011). "Bilobalide inhibits the expression of aquaporin 1, 4 and glial fibrillary acidic protein in rat brain tissue after permanent focal cerebral ischemia." Neural Regeneration Research, 2105-2111. |
|
[4]
|
Teris A. van Beek*, Gerrit P. Lelyveld, "Thin layer chromatography of bilobalide and ginkgolides A, B, C and J on sodium acetate impregnated silica gel." Phytochemical Analysis Volume 4, Issue 3, pages 109-114, May or June 1993 |
|
[5]
|
WANG Xin, L. J. (2012). "Study on Ginkgolides of Wild Ginkgo boliba L. Leaves from Three Gorges Reservoir Areas of Chongqing City." Medicinal Plant, p. 17-19. |
|
[6]
|
Leung, A.Y., & Foster, S. (1996) "Encyclopedia of Common Natural Ingredients Used in Food." Drugs, and Cosmetics, John Wiley & Sons, New York, NY |
|
[7]
|
Bharthi Avula, Yan-Hong Wang, and Troy J. Smille. (2009). "Column Liquid Chromatography/Electrospray Ionization-Time of Flight-Mass Spectrometry and Ultraperformance Column Liquid Chromatography Mass Spectrometry Methods for the Determination of Ginkgolides and Bilobalide in the Leaves of Ginkgo biloba and Dietary Supplements." Journal of AOAC International, pp. 645-652. |
|
[8]
|
Jan Krzek, Janusz S. Czekaj, Wlodzimierz Rzeszutoko and Radoslaw J. Ekiert. (2007)."Validation of Capillary Gas Chromatographic Method for Determination of Bilobalide and Ginkgolides A, B, C in Ginkgo Biloba Dry and Liquid Extracts." Polish Pharmaceutical Society, pp. 303-310 |
|
[9]
|
Stanislav Jaracz, S. M. (2004). "Isolation of ginkgolides A, B, C, J and bilobalide from G. biloba extracts." Phytochemistry, pp. 2897-2902. |
|
[10]
|
Dirk Lichtblau, J. M. (2002). "Efficient Extraction of Ginkgolides and Bilobalide from Ginkgo biloba Leaves." J. Nat. Prod., pp. 1501 - 1504. |
|