|
植物來源 |
|
|
生物活性 |
|
|
鑑定 |
旋光度 |
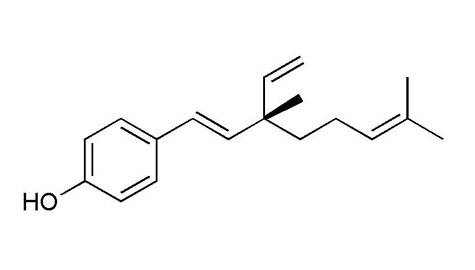
[α]30D +37.2° |
1HNMR


|
| 分析方法 |
|
| 儀器 |
矽膠, HPTLC 板, 1 mm; Merck |
| 流動相 |
20% (v/v) 乙酸乙酯/正己烷 |
| 檢測器 |
UV λ254 nm |
|
|
|
| 儀器 |
Agilent1290 Infinity UPLC 儀 (Agilent Technologies, Santa Clara, CA) |
| 色譜柱 |
Hypersil GOLD C4 色譜柱 (50 mm × 2.1 mm i.d., 粒徑1.9 µm, Thermo Fisher Scientific, Waltham, MA) |
| 流動相 |
A: 0.1% 甲酸水, B: 0.1% 甲酸乙腈, 流率: 0.5 mL/min: 0 min, 30% B; 0.4-1.0 min, 90% B; 1.2 min, 30% B;0.3 min, 重新平衡時間 |
| 檢測器 |
正離子多反應監測ESI (MRM) 模式; 轉讓毛細管溫度: 320°C, 噴射電壓: 4000 V, 鞘氣: 25 psi, m/z 490 → m/z 171.1 |
|
|
|
| 儀器 |
Waters 515 Separations Module HPLC 儀及Waters 2487 二極管數組檢測器 |
| 色譜柱 |
Hypersil ODS2 色譜柱 (250 mm × 4.6 mm i.d., 5 µm; Dalian Elite Analytical Instrument Co. Ltd., Dalian, China) |
| 流動相 |
甲醇: 水 (0.2% formic acid) = 90: 10, v/v;流率: 1.0 mL/min |
| 檢測器 |
UV λ260 nm |
|
|
|
| 儀器 |
泵 (Jasco, PU-2089, Tokyo, Japan) 和UV 檢測器 (Jasco, UV-2075) |
| 色譜柱 |
XTerra™ RP18 色譜柱 (4.6 mm × 250 mm, 5 µm; Waters Co., MA, USA) |
| 流動相 |
A: 0.1% 水-乙酸, B: 乙腈, 0-15 min 60% A, 15-35 min 60-50% A, 35-45 min 50-40% A, 45-55 min 40-30% A, 55-60 min 30-20% A, 1 mL/min |
| 檢測器 |
UV λ245 nm |
|
|
|
| 儀器 |
Agilent 1260 Infinity LC 儀及 DAD 檢測器 |
| 色譜柱 |
Alltima C18 色譜柱 (Alltech, 4.6 × 250 mm, 5 µm) |
| 流動相 |
A: 乙酸水溶液, B: 乙腈, 0-15 min 40-50% B, 15-35 min 50-60% B, 35-55 min 60-80% B, 55-60 min 80% B, 1.0 mL/min |
| 檢測器 |
UV λ251 nm |
|
|
|
| 儀器 |
1100 HPLC 儀 (Agilent Technologies, California, USA) |
| 色譜柱 |
DL-C18 色譜住 (5.0mm, 250 mm, 4.6 mm, Japan) |
| 流動相 |
A: 乙腈, B: 0.01M 甲酸: 0-10 min 15-40% B, 10-30 min 40-55% B, 30-40 min 55-75% B, 40-60 min 75-80% B, 60-70 min 80-95% B, 15% A; 流率: 0.5 mL/min |
| 檢測器 |
UV λ276 nm |
|
| 樣品製備 |
|
方法一 |
|
|
補骨脂 (2.0 kg) 乾燥種子 95% EtOH 提取得 592 g 粗提取, 2.5 L 水中暫停 (392 g). |
|
|
石油醚及乙酸乙酯分餾, 石油醚部中餾分PE (143 g) 和乙酸乙酯中餾分 EA (120 g). |
|
|
餾分 EA (100 g) 上樣矽膠色譜柱 (CC) 以石油醚及提督增加的丙酮洗脫得餾分 1-9, TLC 顯色. 餾分1-4 (共 46 g) 主要含有補骨脂酚。 |
|
|
| 參考文獻 |
|
[1]
|
Zhuang, X., et al. (2013). "Pre-column derivatization combined with UPLC-MS for rapid and sensitive quantification of bakuchiol in rat plasma." Journal of Pharmaceutical and Biomedical Analysis 75(0): 18-24. |
|
[2]
|
Park, E.-J., et al. (2007). "Bakuchiol-induced caspase-3-dependent apoptosis occurs through c-Jun NH2-terminal kinase-mediated mitochondrial translocation of Bax in rat liver myofibroblasts." European Journal of Pharmacology 559(2-3): 115-123. |
|
[3]
|
Chen, Z., et al. (2010). "Anti-tumor effects of bakuchiol, an analogue of resveratrol, on human lung adenocarcinoma A549 cell line." European Journal of Pharmacology 643(2-3): 170-179. |
|
[4]
|
Pae, H.-O., et al. (2001). "Bakuchiol from Psoralea corylifolia inhibits the expression of inducible nitric oxide synthase gene via the inactivation of nuclear transcription factor-κB in RAW 264.7 macrophages." International Immunopharmacology 1(9-10): 1849-1855. |
|
[5]
|
Kim, K.-A., et al. (2013). "Protective effects of the compounds isolated from the seed of Psoralea corylifolia on oxidative stress-induced retinal damage." Toxicology and Applied Pharmacology 269(2): 109-120. |
|
[6]
|
Lim, S. H., et al. (2009). "Ethanol extract of Psoralea corylifolia L. and its main constituent, bakuchiol, reduce bone loss in ovariectomised Sprague-Dawley rats." Br J Nutr 101(7): 1031-1039. |
|
[7]
|
Haraguchi, H., et al. (2002). "Antioxidative components of Psoralea corylifolia (Leguminosae)." Phytother Res 16(6): 539-544. |
|
[8]
|
Miao, L., et al. (2013). "Bakuchiol inhibits androgen induced-proliferation of prostate cancer cell line LNCaP through suppression of AR transcription activity." Tianjin Zhongyiyao 30(5): 291-293. |
|
[9]
|
Katsura, H., et al. (2001). "In vitro antimicrobial activities of bakuchiol against oral microorganisms." Antimicrob Agents Chemother 45(11): 3009-3013. |
|
[10]
|
Katsura, H., et al. (2001). "In vitro antimicrobial activities of bakuchiol against oral microorganisms." Antimicrob Agents Chemother 45(11): 3009-3013. |
|
[11]
|
Yan, D.-M., et al. (2010). "In vivo pharmacokinetics of bakuchiol after oral administration of bakuchiol extraction in rat plasma." Journal of Ethnopharmacology 128(3): 697-702. |
|
[12]
|
Lim, S.-H., et al. (2011). "Estrogenic activities of Psoralea corylifolia L. seed extracts and main constituents." Phytomedicine 18(5): 425-430. |
|
[13]
|
Chen, X., et al. (2013). "Isobavachalcone and bavachinin from Psoraleae Fructus modulate Aβ42 aggregation process through different mechanisms in vitro." FEBS Letters 587(18): 2930-2935. |
|
[14]
|
Chen, Q., et al. (2012). "Separation, identification, and quantification of active constituents in Fructus Psoraleae by high-performance liquid chromatography with UV, ion trap mass spectrometry, and electrochemical detection." Journal of Pharmaceutical Analysis 2(2): 143-151. |
|
[15]
|
Yin, S., et al. (2006). "Psoracorylifols A-E, five novel compounds with activity against Helicobacter pylori from seeds of Psoralea corylifolia." Tetrahedron 62(11): 2569-2575. |
|
| 連結 |
 中藥材圖像數據庫 中藥材圖像數據庫
 藥用植物圖像數據庫 藥用植物圖像數據庫
 中藥標本數據庫 中藥標本數據庫
|
 中藥材圖像數據庫
中藥材圖像數據庫
 藥用植物圖像數據庫
藥用植物圖像數據庫
 中藥標本數據庫
中藥標本數據庫


