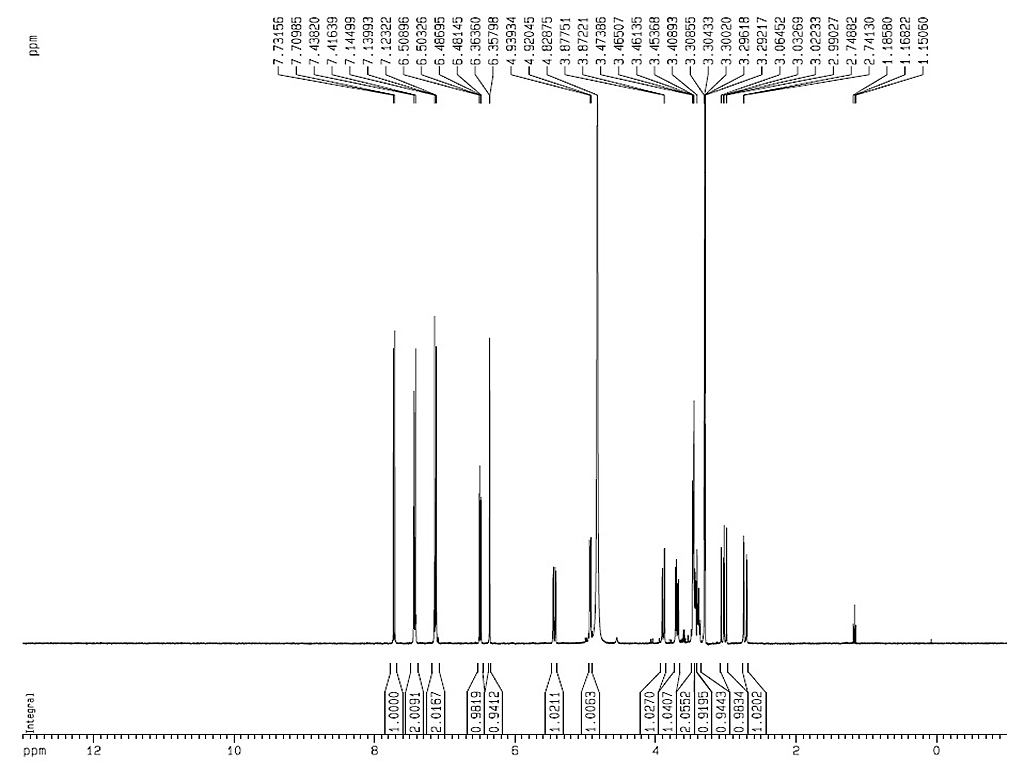
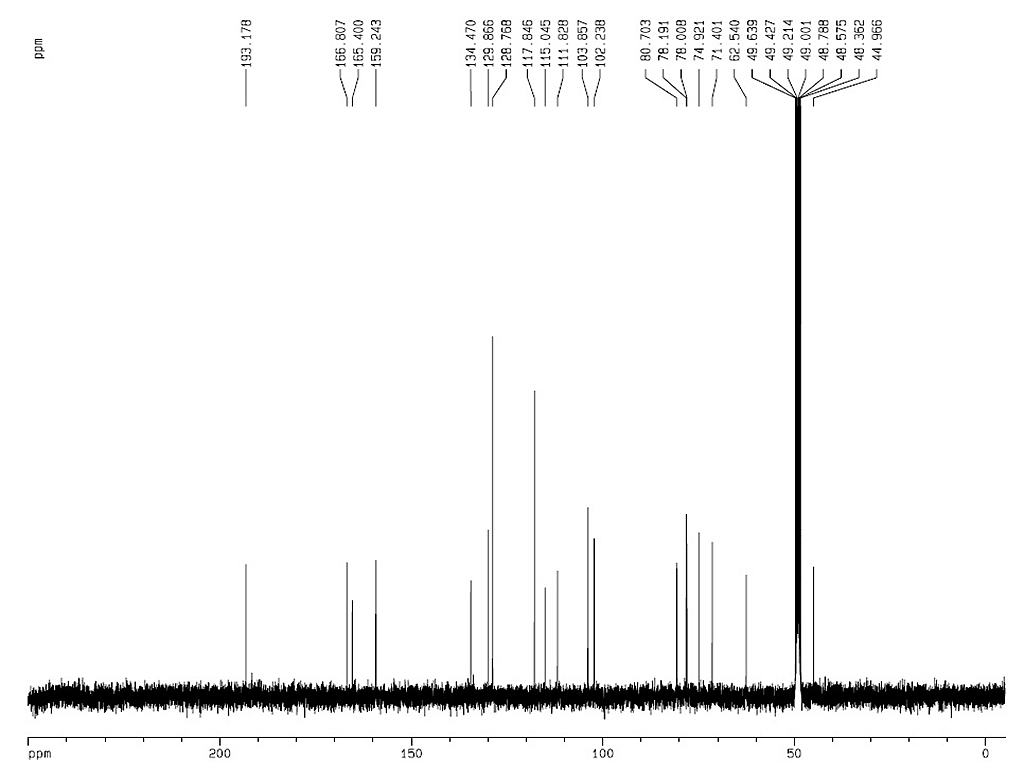
|
Natural Resources |
|
|
Bioactivities |
|
|
Identification |
Melting point |
212°C |
|
|
| Analytical Method |
|
| INSTRUMENT |
Silica gel G plate |
| MOBILE PHASE |
Chloroform: methanol: water = 45: 17: 5, v/v/v |
| DETECTION |
UV365 nm |
|
|
|
| INSTRUMENT |
LC-10ATVP HPLC pump (Shimazdu, Japan) fitted with a SPD-10AVP ultraviolet detector (Shimazdu, Japan) |
| COLUMN |
Diamonsil-C18 250 mm × 4.6 mm I.D. column (packed with 5 µm C18 material, Dikma Company, Beijing, China) |
| MOBILE PHASE |
Glacial acetic acid: water: acetonitrile = 2.3 : 75.7: 22 (v/v/v); flow rate : 1.0 mL/min |
| DETECTION |
UV λ276 nm |
|
|
|
| INSTRUMENT |
HP1100 Agilent Technologies, USA |
| COLUMN |
C18 column (250 mm × 4.6 mm × 5 µm, Agilent Technologies, USA) |
| MOBILE PHASE |
A: acetonitrile, B: 0.5% aqueous acetic acid, 0-11 min 20-40% B, 11-19 min 40% B, 19-20 min 40-20% B; flow rate : 1.0 mL/min. |
| DETECTION |
UV λ276 nm |
|
|
|
| INSTRUMENT |
Agilent 1200LC, U.S.A. |
| COLUMN |
ODS column (250 mm × 4.6 mm, 5 µm, Agilent, U.S.A.), 25°C |
| MOBILE PHASE |
Methanol: water = 45: 55, v/v; 1.0 mL/min |
| DETECTION |
UV λ276 nm |
|
|
|
| INSTRUMENT |
Hewlett-Packard (Waldbronn, Germany) system composed of a HP1100 series binary pump, a HP1100 series photodiode array detector and a HP1100 series autosampler (set at 5 µl) |
| COLUMN |
Wako Wakosil-II 5C18 AR reversed-phase column (particle size of the packing 5 µm, 150 × 4.6 mm i.d.) |
| MOBILE PHASE |
0.01% (v/v) phosphoric acid: acetonitrile = 0 min, 90: 10; 10 min, 88: 12; 22 min, 70: 30; 30 min, 30: 70; flow-rate : 1.0 mL/min |
| DETECTION |
UV λ245 nm |
|
|
|
| INSTRUMENT |
Waters HPLC system consisting of a pump (Model 1525), an auto-sampler (Model 717 plus), UV detector (Waters 2487 Dual λ absorbance detector) |
| COLUMN |
Kromasil-C18 column (5 µm, 4.6 × 250 mm, Akzol Nobel, Sweden) |
| MOBILE PHASE |
Methanol: water: acetic acid = 65: 35: 2, v/v/v; flow rate: 1.0 mL/min |
| DETECTION |
UV λ246 nm |
|
| Sample Preparation |
|
METHOD 1 |
|
|
Pack a 150.0 mg aliquot of PVPB into an empty polypropylene cartridge (Alltech, Deerfield, IL, USA) and precondition with ethanol. Oven-dry, slice and crush the licorice, and then extract 1.0 g of the powder with 25.0 mL methanol for 4 hours. Load 0.2 mL of the obtained extract onto the SPE cartridge. Wash the sample with 3.0 mL of acetonitrile and methanol: water (75: 25, v/v). Elute LQ and glycyrrhizin by methanol and methanol: acetic acid (90: 10, v/v). |
|
|
METHOD 2 |
|
|
Extract 1000 g of G. uralensis roots with deionized water (3 × 3 L) under reflux and repeat 3 times (1 hour each time). Combine the extracts and concentrate under vacuum to afford 340 g aqueous extracts (AE), chromatographe AE on a macroporous resin D101 column (10 × 80 cm, Naikai Chemical Co., China) and elute with deionized water (1 L), 50% ethanol (3 L), 95% ethanol (3 L) to afford three fractions: water fraction (AE1, 122 g), 50% ethanol fraction (AE2, 26.8 g) and 95% ethanol fraction (AE3, 8.83 g). Subject AE2 to a silica gel column (500 g, 5 × 50 cm, 200 mesh, Qingdao Haiyang Chemical Co., China) eluted with chloroform-MeOH (4:1, 3:1, 2:1, 1:1, each 300 ml) affording four subfractions (sfr): sfr.1 to sfr.4. Subject Subfraction 3 to an ODS gel column (1 × 30 cm, 100 mesh, Fuji Silysia Chemical Ltd. Japan), eluted with MeOH-water solution from 10% to 70% with 10% increments, each 100 ml. The 30% MeOH eluant afforded liquiritin (289 mg) |
|
|
| Reference |
|
[1]
|
Wang, W., et al. (2008). "Antidepressant-like effects of liquiritin and isoliquiritin from Glycyrrhiza uralensis in the forced swimming test and tail suspension test in mice." Progress in Neuro-Psychopharmacology and Biological Psychiatry 32(5): 1179-1184. |
|
[2]
|
Ammar, N. M., et al. (2012). "Phytochemical and clinical studies of the bioactive extract of Glycyrrhiza glabra L. Family Leguminosae." Int. J. Phytomed. 4(3): 429-436, 428pp. |
|
[3]
|
Zhao, Z., et al. (2008). "Antidepressant-like effect of liquiritin from Glycyrrhiza uralensis in chronic variable stress induced depression model rats." Behavioural Brain Research 194(1): 108-113. |
|
[4]
|
Ohno, H., et al. (2013). "Evaluation of cytotoxiciy and tumor-specificity of licorice flavonoids based on chemical structure." Anticancer Res. 33(8): 3061-3068. |
|
[5]
|
Zhu, X., et al. (2006). Xuefuzhuyu capsules containing Chinese medicines and its quality control, Tianjin Hongrentang Pharmaceutical Co., Ltd., Peop. Rep. China. 25pp. |
|
[6]
|
Cong, J. and B. Lin (2007). "Separation of Liquiritin by simulated moving bed chromatography." Journal of Chromatography A 1145(1-2): 190-194. |
|
[7]
|
Sun, C., et al. (2007). "Separation of glycyrrhizic acid and liquiritin from licorice root by aqueous nonionic surfactant mediated extraction." Colloids and Surfaces A: Physicochemical and Engineering Aspects 305(1-3): 42-47. |
|
[8]
|
Wang, S., et al. (2013). "Facile optimization for chromatographic separation of liquiritin and liquiritigenin." Journal of Chromatography A 1282(0): 167-171. |
|
[9]
|
Okamura, N., et al. (1999). "Simultaneous high-performance liquid chromatographic determination of puerarin, daidzin, paeoniflorin, liquiritin, cinnamic acid, cinnamaldehyde and glycyrrhizin in Kampo medicines." Journal of Pharmaceutical and Biomedical Analysis 19(3-4): 603-612. |
|
[10]
|
Yang, L., et al. (2013). "Development of sample preparation method for isoliquiritigenin, liquiritin, and glycyrrhizic acid analysis in licorice by ionic liquids-ultrasound based extraction and high-performance liquid chromatography detection." Food Chemistry 138(1): 173-179. |
|
[11]
|
Bi, W., et al. (2010). "Solid-phase extraction of liquiritin and glycyrrhizin from licorice using porous alkyl-pyridinium polymer sorbent." Phytochemical Analysis 21(5): 496-501. |
|
| Link to |
 Chinese Medicinal Material Images Database Chinese Medicinal Material Images Database
 Medicinal Plant Images Database Medicinal Plant Images Database
 Chinese Medicine Specimen Database Chinese Medicine Specimen Database
|
 Chinese Medicinal Material Images Database
Chinese Medicinal Material Images Database
 Medicinal Plant Images Database
Medicinal Plant Images Database
 Chinese Medicine Specimen Database
Chinese Medicine Specimen Database