|
植物來源 |
|
|
生物活性 |
|
|
鑑定 |
熔點 |
337-339°C |
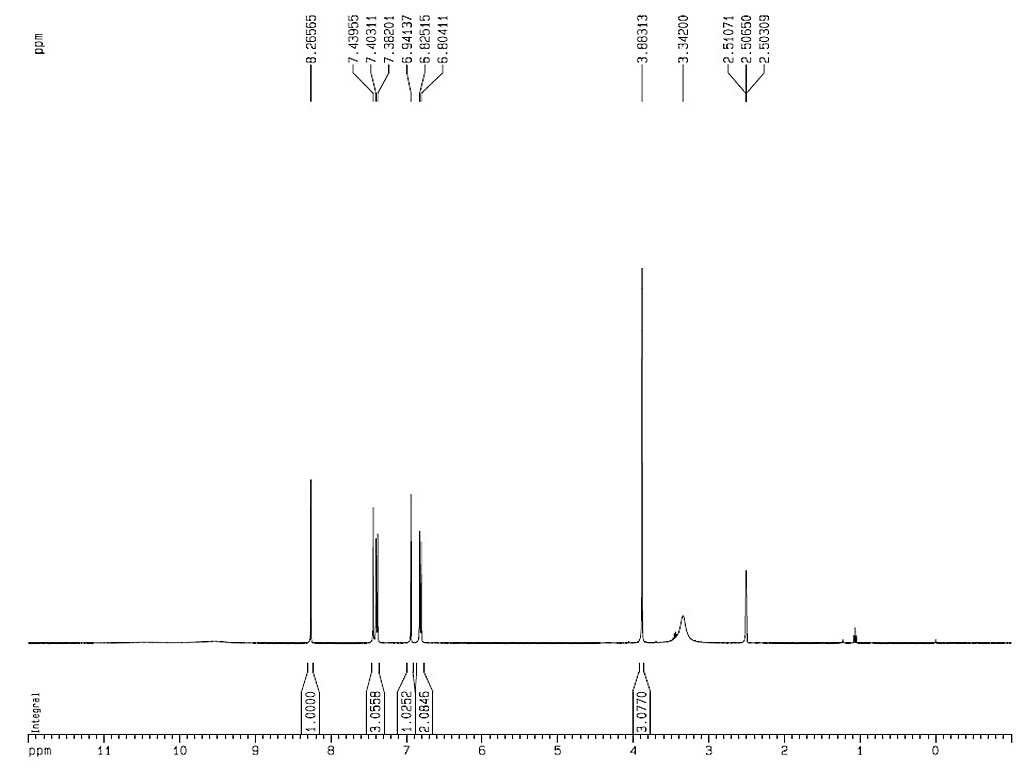
1HNMR

|
| 分析方法 |
|
| 儀器 |
HSGF254 矽膠板 (Yantai Chemical Engineering Institute, China) |
| 流動相 |
甲苯: 乙酸抑制: 丙酮: 甲酸 = 20: 4: 2: 1 |
| 檢測器 |
UV λ254 nm; 濃氨溶液煙霧顯色 |
|
|
|
| 儀器 |
Shimadzu LC-2010AHT HPLC 儀和Shimadzu CLASS-VP 軟件version 6.12 SP4 (Shimadzu Co. Ltd., Japan) |
| 色譜柱 |
Kromasil C18 色譜柱 (4.6 × 250 mm, 5 µm, Tianjin Scientific Instruments Co. Ltd., China), 40°C |
| 流動相 |
A: 0.5% 冰醋酸水溶液, B: 0.5% 冰醋酸甲醇溶液, 0-10 min 30% B, 10-30 min 30-40% B, 30-40 min 40% B, 40-60 min 40-60% B, 進樣量: 25 µL, 1.0 mL/min |
| 檢測器 |
UV λ257.2 nm |
|
|
|
| 儀器 |
Agilent 1200 反相 HPLC 儀 |
| 色譜柱 |
HiQ Sil C18W 反相色譜柱 (250 mm × 4.6 mm i.d., 5 µm), 30°C |
| 流動相 |
甲醇: 水: 甲酸 = 35: 64.935: 0.065 (v/v/v), 1 mL/min, 進樣量: 10 µl |
| 檢測器 |
UV λ260 nm |
|
|
|
| 儀器 |
HP 1200 液相色譜儀, Agilent (Waldbronn, Germany), 配備一個二元泵, 膜脫氣機, 自動進樣器, 和六通閥. |
| 色譜柱 |
Zorbax Eclipse XDB-C18 色譜柱 (50 × 4.6 mm2, 1.8 µm), 25°C |
| 流動相 |
A: 乙腈, B: 0.01% 甲醇水, 0-1.5 min 10% A, 1.5-2.5 min 10-25% A, 2.5-3.5 min 25% A, 3.5-7 min 25-50% A, 7-8 min 50-80% A, 8-10 min 80% A, 10-12 min 80-10% A, 0.5 mL/min, 進樣量: 10 µL |
| 檢測器 |
Triple Quad LC/MS 6410 儀 (Agilent) 配備 ESI 源. ESI-MS 利用正離子多反應監視模式 (MRM), 電噴霧毛細管電壓: 3500 V, 霧化氣壓: 35 psi. 乾燥氣流速: 氮氣12 L/min at 350°C. Agilent Mass Hunter 軟件控制, version B.04.01. |
|
| 樣品製備 |
|
方法一 |
|
|
|
|
|
Type-J 多線圈行星離心機 (P.C. Inc., Potomac, MD, USA). 離心中心軸距離 10 cm. 單 Tefzel 管分離柱 (Zeus Industrial Products) 1.6 mm I.D. (SW 14), 長160 m, 12 層, 2 吋間距. 柱總容量 320 ml. β值內部為 0.5、外部 0.75. 轉速控制器 (Bodine Electric, North Chicago, IL, USA), 800 rpm. UV 檢測器 (Uvicord S, LKB Instruments, Bromma/Stockholm, Sweden). 餾分收集器 (Ultrorac, LKB Instruments). |
|
|
下相為HCl3-MeOH-H2O (4: 3: 2, v/v) |
|
|
2.0 mL/min; 800 rpm |
|
|
UV λ275 nm |
|
|
方法二 |
|
|
負壓空化裝置 (CN2597047), 一個提取和一個收集罐, 氮氣提取, 恒溫控制, 揮發性溶劑冷凝器冷藏。 |
|
|
10 g 樣品置於負壓空化裝置提取, 利用真空泵減壓提取。異黃酮的提取時間, 粒徑, 負壓, 乙醇濃度, 液固比都可被控制。 |
|
|
| 參考文獻 |
|
[1]
|
Zhang, D.-Y., et al. (2010). "Negative pressure cavitation extraction and antioxidant activity of genistein and genistin from the roots of pigeon pea [Cajanus cajan (L.) Millsp.]." Separation and Purification Technology 74(2): 261-270. |
|
[2]
|
Shodehinde, S. A. and G. Oboh (2013). "Antioxidant properties of aqueous extracts of unripe Musa paradisiaca on sodium nitroprusside induced lipid peroxidation in rat pancreas in vitro." Asian Pacific Journal of Tropical Biomedicine 3(6): 449-457. |
|
[3]
|
Lee, J. H., et al. (2013). "Determination of the variations in levels of phenolic compounds in soybean (Glycine max Merr.) sprouts infected by anthracnose (Colletotrichum gloeosporioides)." J Sci Food Agric 93(12): 3081-3086. |
|
[4]
|
Kasper, J. and M. F. Melzig (2011). "HPTLC method for the quantification of isoflavones in nutritional supplements of red clover (Trifolium pratense L.)." J. Planar Chromatogr.--Mod. TLC 24(5): 373-375. |
|
[5]
|
Lengyel, J., et al. (2013). "On the radical scavenging activity of isoflavones: thermodynamics of O-H bond cleavage." Physical Chemistry Chemical Physics 15(26): 10895-10903. |
|
[6]
|
Vitale, D. C., et al. (2013). "Isoflavones: estrogenic activity, biological effect and bioavailability." Eur J Drug Metab Pharmacokinet 38(1): 15-25. |
|
[7]
|
Weng, C. J. and G. C. Yen (2012). "Flavonoids, a ubiquitous dietary phenolic subclass, exert extensive in vitro anti-invasive and in vivo anti-metastatic activities." Cancer Metastasis Rev 31(1-2): 323-351. |
|
[8]
|
Bae, M., et al. (2012). "Inhibitory effects of isoflavonoids on rat prostate testosterone 5alpha-reductase." J Acupunct Meridian Stud 5(6): 319-322. |
|
[9]
|
Hirohata, M., et al. (2012). "Anti-amyloidogenic effects of soybean isoflavones in vitro: Fluorescence spectroscopy demonstrating direct binding to Aβ monomers, oligomers and fibrils." Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 1822(8): 1316-1324. |
|
[10]
|
Masilamani, M., et al. (2012). "Regulation of the immune response by soybean isoflavones." Immunologic Research 54(1-3): 95-110. |
|
[11]
|
Kim, J. H., et al. (2012). "A new isoflavone glycitein 7-O-beta-D-glucoside 4\'-O-methylate, isolated from Cordyceps militaris grown on germinated soybeans extract, inhibits EGF-induced mucus hypersecretion in the human lung mucoepidermoid cells." Phytother Res 26(12): 1807-1812. |
|
[12]
|
Yuan, D., et al. (2006). "TLC and HPLC analysis of soy isoflavones in Semen Sojae Praeparatum." Asian J. Tradit. Med. 1(3-4): 166-172. |
|
[13]
|
Delgado-Zamarreño, M. M., et al. (2012). "A modified QuEChERS method as sample treatment before the determination of isoflavones in foods by ultra-performance liquid chromatography-triple quadrupole mass spectrometry." Talanta 100(0): 320-328. |
|
[14]
|
Yang, F., et al. (2001). "Separation and purification of isoflavones from a crude soybean extract by high-speed counter-current chromatography." Journal of Chromatography A 928(2): 163-170. |
|
| 連結 |
 中藥材圖像數據庫 中藥材圖像數據庫
 藥用植物圖像數據庫 藥用植物圖像數據庫
|
 中藥材圖像數據庫
中藥材圖像數據庫
 藥用植物圖像數據庫
藥用植物圖像數據庫


