|
[1]
|
Xu, K., et al. (2013). "GC-MS analysis on chemical constituents of volatile oils in different fractions of Isodon amethystoides." Zhonghua Zhongyiyao Xuekan 31(8): 1797-1799. |
|
[2]
|
Wang, Q., et al. (2013). "GC-MS analysis of the low-polarity chemical constituents from Parochetus communis Buch." Med. Plant 4(9): 50-51, 55. |
|
[3]
|
Tang, B.-q., et al. (2013). "Chemical constituents in leaves of Morus atropurpurea and their α-glucosidase activity." Zhongcaoyao 44(22): 3109-3113. |
|
[4]
|
El-Domiaty, M. M., et al. (2013). "Phytochemical and biological investigation of Lagenaria siceraria (Molina) Standl. cultivated in Egypt." Biosci., Biotechnol. Res. Asia 10(2): 533-550. |
|
[5]
|
Zhong, R. M., et al. (2013). "Antimicrobial activity of Myrica rubra essential oil against five pathogenic food-borne bacteria." Adv. Mater. Res. (Durnten-Zurich, Switz.) 781-784(Advances in Chemical Engineering III): 1646-1651, 1647pp. |
|
[6]
|
Sharma, R., et al. (2014). "The potential of Leucosidea sericea against Propionibacterium acnes." Phytochem. Lett. 7: 124-129. |
|
[7]
|
Feng, C., et al. (2008). "Chemical constituents of medicinal mangrove plant Hibiscus tiliaceus." Haiyang Kexue 32(9): 57-60. |
|
[8]
|
Yang, H.-k., et al. (2013). "An analysis on the constituents of the volatile oil from different parts of Ardisia mamillata." Jiangxi Nongye Daxue Xuebao 35(5): 993-998. |
|
[9]
|
Wei, H., et al. (2013). "Chemical constituents in leaves of Cyclosorus parasiticus." Zhongcaoyao 44(17): 2354-2357. |
|
[10]
|
Costa, J. P., et al. (2014). "Anxiolytic-like effects of phytol: Possible involvement of GABAergic transmission." Brain Res. 1547: 34-42. |
|
[11]
|
Costa, J. P., et al. (2012). "Anticonvulsant effect of phytol in a pilocarpine model in mice." Neurosci. Lett. 523(2): 115-118. |
|
[12]
|
Saikia, D., et al. (2010). "Antitubercular potential of some semisynthetic analogues of phytol." Bioorg. Med. Chem. Lett. 20(2): 508-512. |
|
[13]
|
Goto, T., et al. (2005). "Phytol directly activates peroxisome proliferator-activated receptor α (PPARα) and regulates gene expression involved in lipid metabolism in PPARα-expressing HepG2 hepatocytes." Biochemical and Biophysical Research Communications 337(2): 440-445. |
|
[14]
|
Jegadeeswari, P., et al. (2012). "GC-MS analysis of bioactive components of Aristolochia krysagathra (Aristolochiaceae)." J. Curr. Chem. Pharm. Sci. 2(4): 226-232. |
|
[15]
|
Kulandai Therese, N., et al. (2012). "GC-MS analysis of bioactive constituents of Hedyotis leschenaultiana DC (Rubiaceae)." Int. J. Appl. Biol. Pharm. Technol. 3(4): 159-164. |
|
[16]
|
Paranthaman, R., et al. (2012). "GC-MS analysis of phytochemicals and simultaneous determination of flavonoids in Amaranthus caudatus (Sirukeerai) by RP-HPLC." J. Anal. Bioanal. Tech. 3(5): 1000147/1000141-1000147/1000144. |
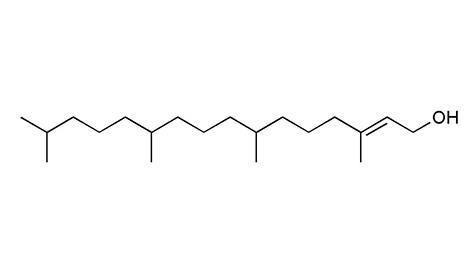
 Medicinal Plant Images Database
Medicinal Plant Images Database