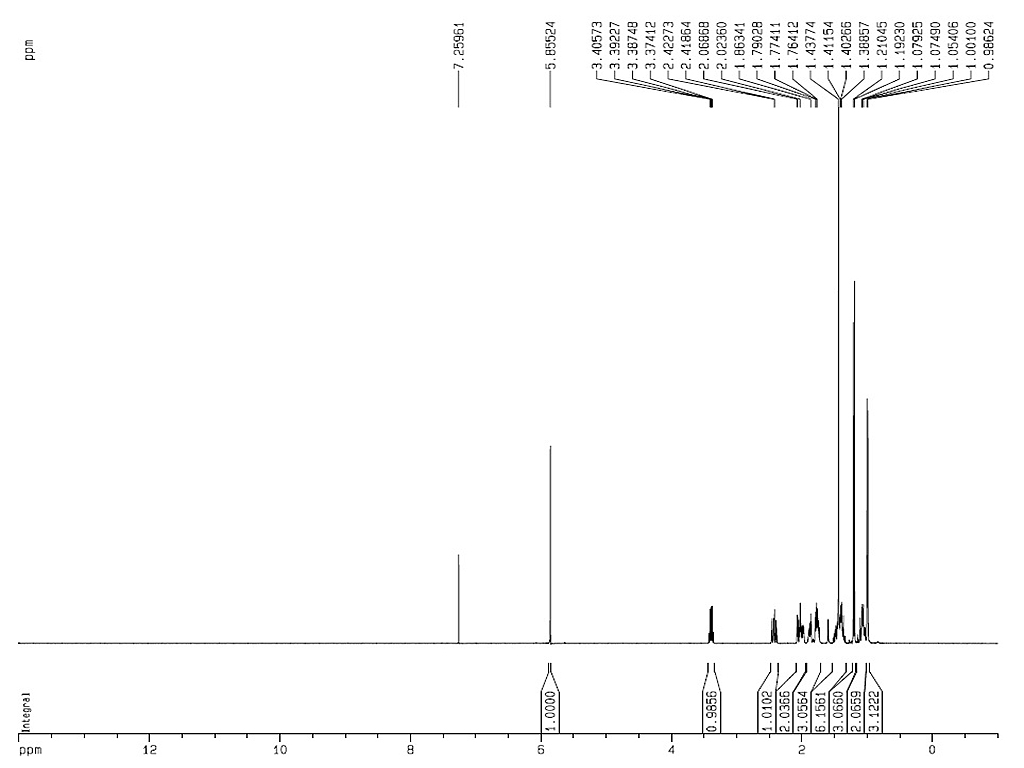
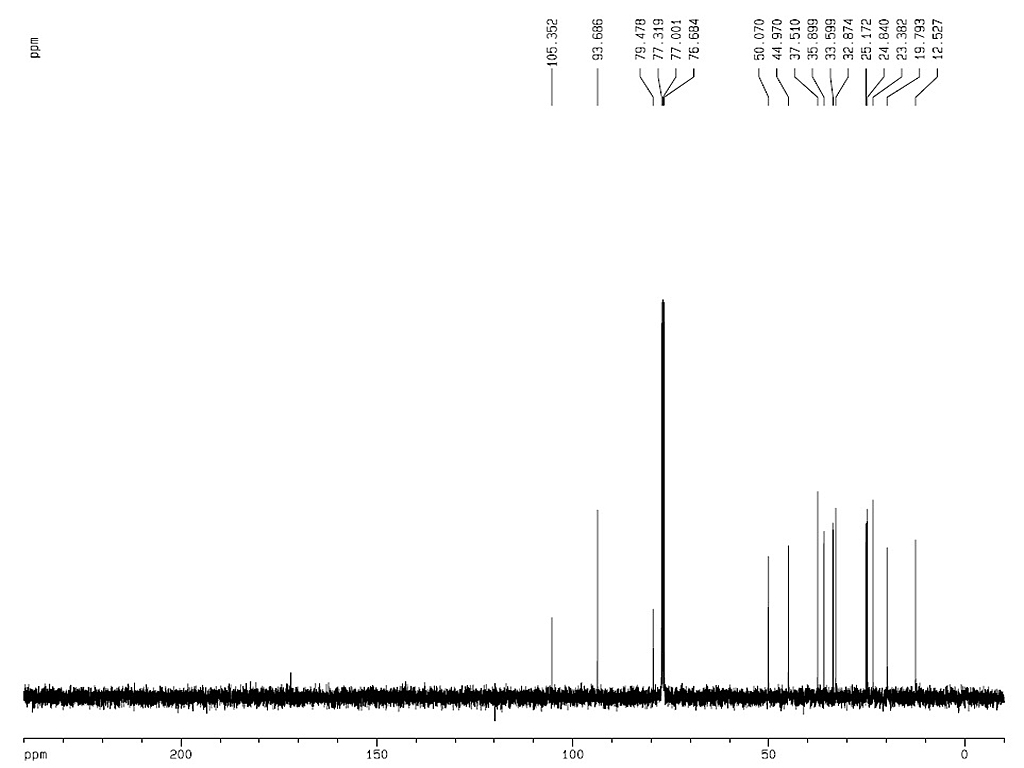
|
Natural Resources |
|
|
Bioactivities |
|
|
Identification |
Melting point |
152-157°C |
| Optical rotation |
[α]20D+64 to +66° (C=2.0g/100ml, chloroform) |
|
|
| Analytical Method |
|
| INSTRUMENT |
RP-C18 thin-layer plate |
| MOBILE PHASE |
Chloroform: methanol = 60: 10 v/v |
| DETECTION |
UV λ215 nm |
|
|
|
| INSTRUMENT |
Varian Prostar equipped with a variable UV wavelength detector, autosampler, and a column thermostat. Operation and data acquisition were carried out through the Varian MS workstation Ver. 6.8 (Varian Associates, Palo Alto, CA, USA) |
| COLUMN |
C-18 column (150 × 4.6 mm, 5 μm) (LUNA Phenomenex, USA) |
| MOBILE PHASE |
Acetonitrile: 0.1% acetic acid = 60: 40, 1.0 mL /min |
| DETECTION |
UV λ254 nm |
|
|
|
| INSTRUMENT |
Hitachi (Hitachi Technologies, Atlanta, GA) composed of a L-7100 gradient quaternary pump equipped with a degasser, a L-7250 programmable auto sampler, and a PL-ELS1000 ELSD (Polymer Laboratories, Amherst, MA), with the data collection through the Hitachi D-7000 HPLC System Manager software. |
| COLUMN |
Endcapped Purosphere (Hitachi Technologies, Atlanta, GA) C18-RP 250 mm × 4.0 mm ID (5.0 × m pore size) |
| MOBILE PHASE |
A: isocratic and constituted of water, adjusted to pH 3.0-3.5 with trifluoro acetic acid (TFA), B: acetonitrile (65: 35), 1.0 mL/min |
| DETECTION |
CONDITIONS ON THE ELSD: evaporative temperature of 80°C, nebulizer at 75°C, and nitrogen flow at 0.8 L/min |
|
|
|
| INSTRUMENT |
GC-2010 (Shimadzu, Columbia, MD) equipped with a flame ionization detector, with the data collection through the Shimadzu GC-Solution software. |
| COLUMN |
Rtx-5 crossbond 100% dimethyl polysiloxane (Resteck Corp), (15 m × 0.25 mm ID, 0.25 × m film thickness). Column temperature was set at 195°C, injector at 240°C, and FID temperature set at 300°C, and sampling rate of 40 ms. |
| MOBILE PHASE |
Helium as the carrier gas: pressure: 15.6 psi, total flow: 37.4 mL/min, column flow: 1.50 mL/min, linear velocity: 50.5 cm/s, purge flow: 3.0 mL/min, and a split ratio of 21: 9 |
| DETECTION |
FID temperature : 300°C, sampling rate : 40 ms |
|
| Sample Preparation |
|
METHOD 1 |
|
|
50 mL of the concentrated ethanolic extract of A. annua was coated evenly with 16 g of diatomite. The slurry was dried with a rotary evaporator at 40°C at 8 mbar, then ground into a fine powder and packed into a 3.3 cm × 40 cm glass column. Column was then eluted with 200 mL of a mixture of ethyl acetate and hexane (15: 85). Additional 30 mL of hexane was added to the eluate after which the solution was filtered through 8 g of silica gel. |
|
|
The filtrate was evaporated by rotary evaporator after which 40 mL of hexane was used to re-dissolve the dried material. The liquid was stored at 4°C overnight to allow for free crystallization, followed by filtering off the crude ART crystals. The crude crystals were washed four times with a 10% ethyl acetate in hexane mixture. The remaining mother liquor was placed in the fridge and ART was allowed to crystallize after which it was recovered and washed as previously described. The combined crystals were dissolved and dried completely. |
|
|
Using hot filtration with methanol and water. The semi-purified ART was re-dissolved in hot methanol. Hot water was then added dropwise into the clear ART solution until the solution became turbid. A small amount of methanol was then added to clear the solution again. The solution was decolorized by the addition of 50 mg of activated charcoal and filtered immediately at around 80°C through a warm glass frit covered with 1 g of diatomite on the filter bed. The resulting clear ART filtrate was allowed to slowly evaporate and white needle-shaped ART crystals were obtained. |
|
|
METHOD 2 |
|
|
A batch of 65 g of Artemisia powder added with added 7% ethyl alcohol evenly loaded inside a 250 ml stainless steel tubular extractor, packing intermittently with seven 1 cm-thickness glass wool layers. Soaked for 2 hours in the extractor prior to the desired extractive condition reached. Rest ethyl alcohoL added into a 10 mL on-line cartridge and flowed into the extractor carried by the SC-CO2 fluid. |
|
|
OPERATION: Liquid CO2 from a siphon-tube type cylinder (1) went through a cooling bath at 283 K and then compressed to the desired working pressure by a syringe pump (4) and heated up to the supercritical condition by a coil-type heat exchanger (7). The supercritical CO2 flowed into an extractor (8), contacted with powders, and extracted artemisinin and scopoletin into the CO2 phase. Temperature controllers (5), temperature detector (20), and pressure gauge (5) were used to manipulate and control the desired temperature and pressure. During the extraction, the extract laden CO2 gas led into the first absorber (13) filled with 500 mL ethanol by a pressure decrease. Then, the extract was further collected in the second separator filled with 500 mL ethanol controlled at 5 MPa and 303 K. Finally, the expanded low-pressure CO2 gas released from the extract passed through a wet gas flow meter (16) and went to the ambient condition. The precipitates inside the back pressure regulator was washed out; mixed with the extract absorbed in ethanol; dried out the solvent; dissolved in 2 ml absolute ethanol ready for the GC and HPLC analyses of artemisinin. The 50 ml samples were taken in triplexes; the solvent was dried out in a force circulation oven at 60°C (333 K); the residue was weighed by a Mettler balance ready for the determination of the purity, the recovery and the yield. |
|
|
| Reference |
|
[1]
|
Park, K. H., et al. (2012). "Artemisinin inhibits lipopolysaccharide-induced interferon-β production in RAW 264.7 cells: Implications on signal transducer and activator of transcription-1 signaling and nitric oxide production." International Immunopharmacology 14(4): 580-584. |
|
[2]
|
Mueller, M. S., et al. (2000). "The potential of Artemisia annua L. as a locally produced remedy for malaria in the tropics: agricultural, chemical and clinical aspects." J Ethnopharmacol 73(3): 487-493. |
|
[3]
|
Firestone, G. L. and S. N. Sundar (2009). "Anticancer activities of artemisinin and its bioactive derivatives." Expert Rev Mol Med 11: e32. |
|
[4]
|
Xiong, Z., et al. (2010). "Artemisinin, an anti-malarial agent, inhibits rat cardiac hypertrophy via inhibition of NF-κB signaling." European Journal of Pharmacology 649(1-3): 277-284. |
|
[5]
|
Kim, J.-T., et al. (2002). "In vitro antiprotozoal effects of artemisinin on Neospora caninum." Veterinary Parasitology 103(1-2): 53-63. |
|
[6]
|
Ploypradith, P. (2004). "Development of artemisinin and its structurally simplified trioxane derivatives as antimalarial drugs." Acta Tropica 89(3): 329-342. |
|
[7]
|
Adegoke, O., et al. (2012). "Highly sensitive liquid chromatographic analysis of artemisinin and its derivatives after pre-column derivatization with 4-carboxyl-2,6-dinitrobenzene diazonium ion." Acta Chromatographica 24(3): 445-462. |
|
[8]
|
Peng, C. A., et al. (2006). "Direct analysis of artemisinin from Artemisia annua L. using high-performance liquid chromatography with evaporative light scattering detector, and gas chromatography with flame ionization detector." J Chromatogr A 1133(1-2): 254-258. |
|
[9]
|
Liu, N. Q., et al. (2011). "A novel purification method of artemisinin from Artemisia annua." Industrial Crops and Products 34(1): 1084-1088. |
|
[10]
|
Tzeng, T.-C., et al. (2007). "EXTRACTION of scopoletin and artemisinin from Artemisia annua L." Separation and Purification Technology 56(1): 18-24. |
|
[11]
|
Gabriels, M. and J. Plaizier-Vercammen (2004). "Development of a reversed-phase thin-layer chromatographic method for artemisinin and its derivatives." J. Chromatogr. Sci. 42(7): 341-347. |
|
| Link to |
 Chinese Medicinal Material Images Database Chinese Medicinal Material Images Database
 Medicinal Plant Images Database Medicinal Plant Images Database
 Chinese Medicine Specimen Database Chinese Medicine Specimen Database
|
 Chinese Medicinal Material Images Database
Chinese Medicinal Material Images Database
 Medicinal Plant Images Database
Medicinal Plant Images Database
 Chinese Medicine Specimen Database
Chinese Medicine Specimen Database