|
植物来源 |
|
|
生物活性 |
|
|
鉴定 |
熔点 |
101-102°C |
1HNMR

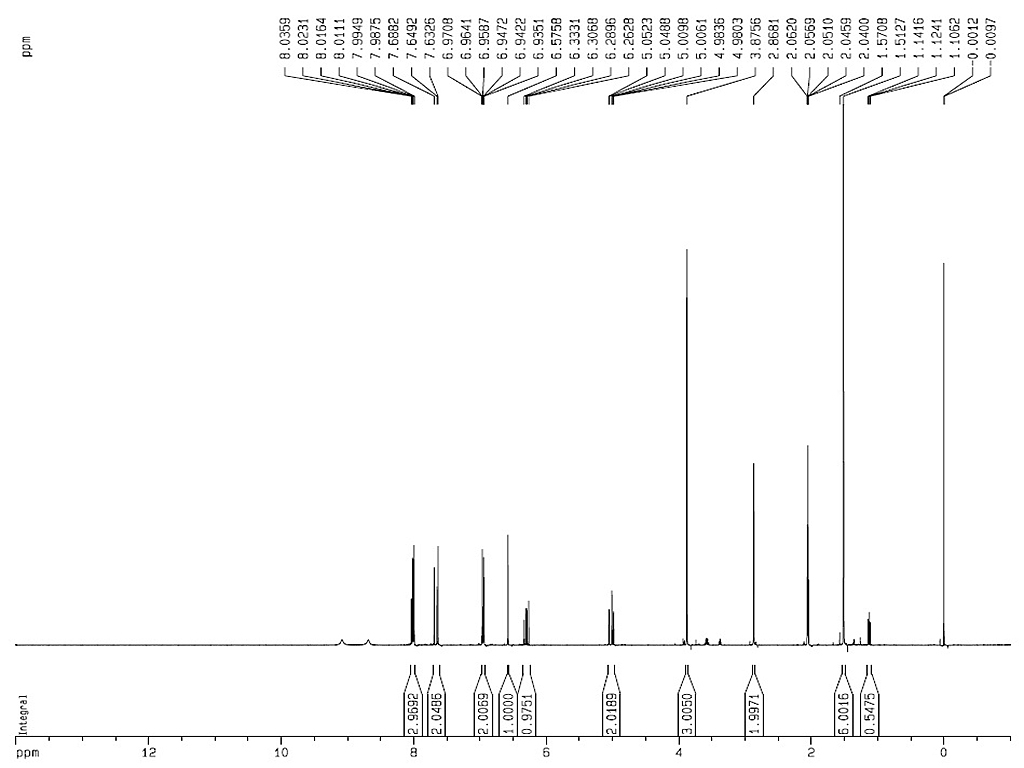
|
| 分析方法 |
|
| 仪器 |
预制60 F254 硅胶板, 0.2 mm thick (Merck) |
| 流动相 |
乙酸乙酯: 己烷: 甲酸 = 4: 4: 0.1 (v/v) 或正己烷: 乙酸乙酯: 乙酸 = 60: 45: 0.7 (v/v) |
| 检测器 |
10% 硫酸喷淋加热显色 |
|
|
|
| 仪器 |
CAMAG Linomat 5 半自动进样器; 聚酰胺膜 |
| 流动相 |
0.23 mol/L SDS: 16% 正己烷: 11% 甲醇: 2.4% 正庚烷: 水 |
| 检测器 |
UV λ366 nm |
|
|
|
| 仪器 |
Hitachi Model D-2000 HPLC仪 |
| 色谱柱 |
TSKgel-C18 ODS-100Z 色谱柱 (150 mm × 4.6 mm, 5 µm) |
| 流动相 |
A: 乙腈, B: 0.1% 甲酸, 0-10 min 15-20% A, 10-12 min 20-23% A, 12-26 min 23-23.7% A, 26-30 min 23.7-32% A, 30-50 min 32-52% A, 50-58 min 52-55% A, 58-63 55% A, 63-70 min 55-56% A, 70-85 min 56-80% A, 1.0mL/min |
| 检测器 |
UV λ280 nm |
|
|
|
| 仪器 |
HP1100 HPLC 仪 (Agilent, USA) |
| 色谱柱 |
Kromasil KR100-5C18 色谱柱, 150 mm × 4.6 mm i.d., Dalian Elite Analytical Instruments |
| 流动相 |
A: 乙腈, B: 0.05% 三氟乙酸, 0-5 min 20-40% A, 5-10 min 40-50% A, 10-25 min 50% A, 25-35 min 50-80% A, 0.8 mL/min |
| 检测器 |
UV λ254 nm 合 364 nm |
|
|
|
| 仪器 |
An Agilent 1200 HPLC 系统 (Agilent Technologies, Waldbronn, Germany), 包括二元泵, 二极管数组检测器, 自动进样器, 恒温柱温箱 |
| 色谱柱 |
Agilent Zorbax SB-C18 色谱柱 (50 × 4.6 mm, 1.8 µm), 30°C |
| 流动相 |
A: HCOOH: H2O (0.2: 100, v/v), B: ACN, 0-4 min 19% B, 4-11 min 19-27% B, 11-17 min 27-38% B, 17-23 min 38-55% B, 23-25 min 55% B, 25-28 min 55-65% B, 28-30 min 65% B, 30-36 min 65-100%, 0.7 mL/min, 进样量: 1 µL |
| 检测器 |
UV λ190 nm 至 400 nm; Agilent 6530 Q-TOF 质谱仪及 ESI 接口和 Agilent MassHunter Acquisition 软件Ver. A.01.00, MassHunter 工作站Version B.02.00, 干燥气: N2, 柳绿: 5.0 L/min, 干燥气温: 325°C, 雾化气: 45 psig, 鞘气温度: 400°C, 鞘气流: 12 L/min, 毛细管: 3500 V, 撇渣器: 65 V, OCT RF V: 750 V, 碎片: 100 V, 碰撞能: 5-35 V, 正负离子模式, 质谱范围: m/z 100-1700 |
|
| 样品制备 |
|
方法一 |
|
|
乙醇-水 (95: 5) SK3200LH 超声仪超声提取三次 (Shanghai Kudos Ultrasonic Instrument Co., Shanghai, China). Model SENCO R-201 旋转蒸发器合并浓缩 (Shanghai Shensheng Biotech Co., Shanghai, China). 2% 氢氧化钠溶解, 真空过滤. Hangxhou Xinhua Paper Industry (Hangzhou, China) 滤纸过滤. 2% 盐酸溶液酸化. 冷水冲洗, Model FD-1 冻干机冻干 (Beijing Boyikang Technology, Beijing, China). HSCCC 分离: 下相正己烷-氯仿-甲醇-水 (5: 6: 3: 2, v/v) 流动相1.8 mL/min, 800 rpm, UV 254 nm. 收集馏分, HSCCC 再纯化. |
|
|
Model TBE-300A HSCCC 仪, Tauto Biotech Co., Shanghai, China 配备恒温外套的多层螺旋星式离心, Model S1007 恒流泵 (Beijing Shengyitong Technology Development, Beijing, China), Model 8823A UV 检测器 (Beijing Institute of New Technology Application), 手动进样阀及 20 ml 回路, Model 3057 便携收集器 (Sichuan Instrument Factory, Chongqing, China) 和 Model Sepu3000 色谱数据工作站Hangzhou Puhui Scientific Technology. |
|
|
下相正己烷-氯仿-甲醇-水 (1.5: 6: 3: 2, v/v) |
|
|
1.5mL/min; 800rpm |
|
|
UV λ254 nm |
|
|
方法二 |
|
|
样品粉末 70% 乙醇提取三次, 2 小时. 过滤, 蒸干. |
|
|
水中溶, CH2Cl2, EtOAc, 和 n-butanol 分离. DPPH-HPLC-MS 筛选分离. EtOAc 部备用. |
|
|
EtOAc 提取物过硅胶柱 (2.0 kg, 100-200 mesh, 10 × 100 cm), CH2Cl2-MeOH (60:1 - 1:1, v/v) 为流动相. 馏分 34-41, CH2Cl2-MeOH = 30: 1 (v/v) 洗脱, 过硅胶柱 (200 g, 200-300 mesh, 3.5 × 60 cm) 石油醚-EtOAc 梯度洗脱 (10:1 - 1:1, v/v). 馏分75-95 结晶, CH2Cl2-MeOH (2:1, v/v) 洗脱, 甲醇中得目标化合物. |
|
|
方法三 |
|
|
乙酸乙酯室温提取三次. 合并, 真空浓缩. |
|
|
乙酸乙酯溶解, 以正己烷洗脱分离. 过硅胶柱 (5.0 × 45.0 cm) 乙酸乙酯/正己烷 (1: 2) 得到 8 馏分 (A-H). |
|
|
馏分 H 过 RP-18 硅胶柱, 甲醇/水洗脱 (1.5: 1) UV 检测, 285 nm 流速 5 mL/min. |
|
|
| 参考文献 |
|
[1]
|
Fu, Y., et al. (2013). "Antioxidant and anti-inflammatory activities of six flavonoids separated from licorice." Food Chemistry 141(2): 1063-1071. |
|
[2]
|
Furuhashi, I., et al. (2005). "Inhibition by licochalcone A, a novel flavonoid isolated from liquorice root, of IL-1beta-induced PGE2 production in human skin fibroblasts." J Pharm Pharmacol 57(12): 1661-1666. |
|
[3]
|
Chu, X., et al. (2013). "Attenuation of allergic airway inflammation in a murine model of asthma by Licochalcone A." Immunopharmacol Immunotoxicol. |
|
[4]
|
Chu, X., et al. (2012). "Licochalcone a inhibits lipopolysaccharide-induced inflammatory response in vitro and in vivo." J Agric Food Chem 60(15): 3947-3954. |
|
[5]
|
Rafi, M. M., et al. (2000). "Modulation of bcl-2 and cytotoxicity by licochalcone-A, a novel estrogenic flavonoid." Anticancer Res 20(4): 2653-2658. |
|
[6]
|
Yuan, X., et al. (2013). "Licochalcone a-induced human bladder cancer t24 cells apoptosis triggered by mitochondria dysfunction and endoplasmic reticulum stress." Biomed Res Int 2013: 474272. |
|
[7]
|
Kim, Y. J., et al. (2013). "Licochalcone A Enhances Geldanamycin-Induced Apoptosis through Reactive Oxygen Species-Mediated Caspase Activation." Pharmacology 92(1-2): 49-59. |
|
[8]
|
Xiao, X.-y., et al. (2011). "Licochalcone A inhibits growth of gastric cancer cells by arresting cell cycle progression and inducing apoptosis." Cancer Letters 302(1): 69-75. |
|
[9]
|
Fu, Y., et al. (2004). "Licochalcone-A, a novel flavonoid isolated from licorice root (Glycyrrhiza glabra), causes G2 and late-G1 arrests in androgen-independent PC-3 prostate cancer cells." Biochemical and Biophysical Research Communications 322(1): 263-270. |
|
[10]
|
Kim, Y. H., et al. (2010). "Antiangiogenic effect of licochalcone A." Biochem Pharmacol 80(8): 1152-1159. |
|
[11]
|
Li, Y.-J., et al. (2011). "Screening and characterization of natural antioxidants in four Glycyrrhiza species by liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry." Journal of Chromatography A 1218(45): 8181-8191. |
|
[12]
|
Hao, H., et al. (2013). "Effect of Licochalcone A on Growth and Properties of Streptococcus suis." PLoS One 8(7): e67728. |
|
[13]
|
Kim, S. N., et al. (2008). "Licochalcone A inhibits the formation and bone resorptive activity of osteoclasts." Cell Biology International 32(9): 1064-1072. |
|
[14]
|
Won, S.-R., et al. (2007). "Licochalcone A: A lipase inhibitor from the roots of Glycyrrhiza uralensis." Food Research International 40(8): 1046-1050. |
|
[15]
|
Cui, S., et al. (2007). "Identification of Radix Glycyrrhizae by microemulsion thin-layer chromatography." Zhongcaoyao 38(4): 540-542. |
|
[16]
|
Zhang, J., et al. (2012). "Flavonoids content in Licorice residue determined by HPLC." Zhongguo Zhongyiyao Keji 19(3): 233-234. |
|
[17]
|
Wang, Q.-E., et al. (2004). "Isolation and purification of inflacoumarin A and licochalcone A from licorice by high-speed counter-current chromatography." Journal of Chromatography A 1048(1): 51-57. |
|
| 连结 |
 中药材图像数据库 中药材图像数据库
 药用植物图像数据库 药用植物图像数据库
 中药标本数据库 中药标本数据库
|
 中药材图像数据库
中药材图像数据库
 药用植物图像数据库
药用植物图像数据库
 中药标本数据库
中药标本数据库


