|
Natural Resources |
|
|
Bioactivities |
|
|
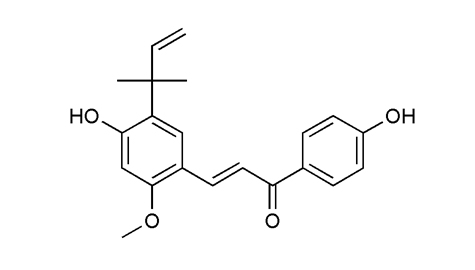
Identification |
Melting point |
101-102°C |
1HNMR

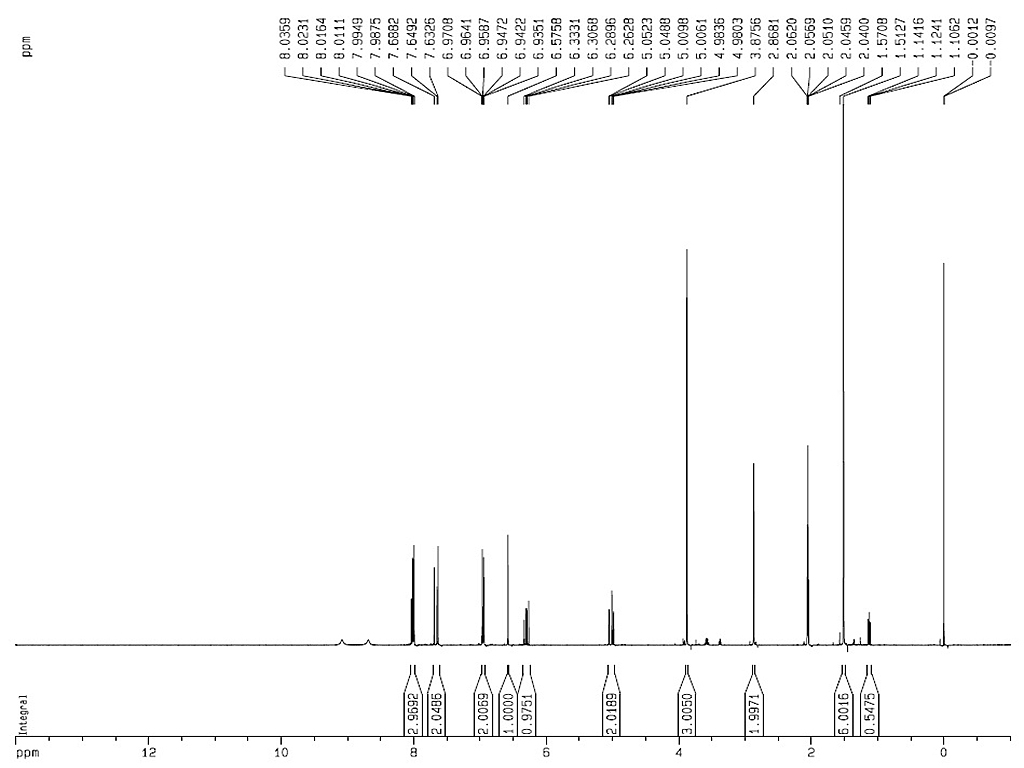
|
| Analytical Method |
|
| INSTRUMENT |
Precoated Kieselegel 60 F254 plates, 0.2 mm thick (Merck) |
| MOBILE PHASE |
Ethyl acetate: hexane: formic acid = 4: 4: 0.1 (v/v) or n-hexane: ethyl acetate: acetic acid = 60: 45: 0.7 (v/v) |
| DETECTION |
10% sulfuric acid reagent and heating |
|
|
|
| INSTRUMENT |
CAMAG Linomat 5 semi-automatic sampler; polyamide film |
| MOBILE PHASE |
0.23 mol/L SDS: 16% n-butanol: 11% methanol: 2.4% n-heptane: water |
| DETECTION |
UV λ366 nm |
|
|
|
| INSTRUMENT |
Hitachi Model D-2000 HPLC |
| COLUMN |
TSKgel-C18 ODS-100Z column (150 mm × 4.6 mm, 5 µm) |
| MOBILE PHASE |
A: Acetonitrile, B: 0.1% formic acid solution, 0-10 min 15-20% A, 10-12 min 20-23% A, 12-26 min 23-23.7% A, 26-30 min 23.7-32% A, 30-50 min 32-52% A, 50-58 min 52-55% A, 58-63 55% A, 63-70 min 55-56% A, 70-85 min 56-80% A, 1.0mL/min |
| DETECTION |
UV λ280 nm |
|
|
|
| INSTRUMENT |
HP1100 HPLC instrument (Agilent, USA) |
| COLUMN |
Kromasil KR100-5C18, 150 mm × 4.6 mm i.d., Dalian Elite Analytical Instruments |
| MOBILE PHASE |
A: acetonitrile, B: 0.05% aqueous trifluoroacetic acid, 0-5 min 20-40% A, 5-10 min 40-50% A, 10-25 min 50% A, 25-35 min 50-80% A, 0.8 mL/min |
| DETECTION |
UV λ254 nm and 364 nm |
|
|
|
| INSTRUMENT |
An Agilent 1200 HPLC system (Agilent Technologies, Waldbronn, Germany) with a binary pump, diode array detection (DAD) system, an auto plate-sampler, and a thermostatically controlled column compartment |
| COLUMN |
Agilent Zorbax SB-C18 column (50 × 4.6 mm, 1.8 µm), 30°C |
| MOBILE PHASE |
A: HCOOH: H2O (0.2: 100, v/v), B: ACN, 0-4 min 19% B, 4-11 min 19-27% B, 11-17 min 27-38% B, 17-23 min 38-55% B, 23-25 min 55% B, 25-28 min 55-65% B, 28-30 min 65% B, 30-36 min 65-100%, 0.7 mL/min, injection volume: 1 µL |
| DETECTION |
UV λ190 nm to 400 nm; Agilent 6530 Q-TOF MS with an ESI interface and Agilent MassHunter Acquisition Software Ver. A.01.00 operated under MassHunter Workstation Software Version B.02.00, drying gas: N2, flow rate: 5.0 L/min, drying gas temperature: 325°C, nebulizer: 45 psig, sheath gas temperature: 400°C, sheath gas flow: 12 L/min, capillary: 3500 V, skimmer: 65 V, OCT RF V: 750 V, fragmentor voltage: 100 V, sample collision energy: 5-35 V, both positive and negative modes, mass spectra range: m/z 100-1700 |
|
| Sample Preparation |
|
METHOD 1 |
|
|
Extract the roots of Glycyrrhiza inflata Bat. three times with ethanol-water (95: 5) by sonication using a SK3200LH ultrasonic cleaning instrument (Shanghai Kudos Ultrasonic Instrument Co., Shanghai, China). Combine and concentrate the extracts under reduced pressure with a Model SENCO R-201 rotary evaporator (Shanghai Shensheng Biotech Co., Shanghai, China). Dissolve the residue in 2% aqueous sodium hydroxide and filter it under reduced pressure in a vacuum filtration devise. Filter by two sheets of double-ring brand cellulose qualitative filter paper manufactured by Hangxhou Xinhua Paper Industry (Hangzhou, China). Acidify the filtrate with 2% aqueous hydrochloric acid until sedimentation process stops. Wash the sediment with cool water and freeze-dry with a Model FD-1 freezing drier (Beijing Boyikang Technology, Beijing, China) to get the crude extract. Run HSCCC to separate the crude extract under following condition: lower phase of n-hexane-chloroform-methanol-water (5: 6: 3: 2, v/v) as mobile phase at 1.8 mL/min, 800 rpm and detected at UV 254 nm. Collect the fractions for purification by HSCCC again. |
|
|
Model TBE-300A HSCCC system manufactured by Tauto Biotech Co., Shanghai, China with a thermostatic jacket to keep the multi-layer coil planet centrifuge at constant temperature, a Model S1007 constant-flow pump (Beijing Shengyitong Technology Development, Beijing, China), a Model 8823A UV detector (Beijing Institute of New Technology Application), a manual sample injection valve with a 20 ml loop, a Model 3057 portable recorder (Sichuan Instrument Factory, Chongqing, China) and a Model Sepu3000 chromatographic data station provided by Hangzhou Puhui Scientific Technology. |
|
|
Lower phase of n-hexane-chloroform-methanol-water (1.5: 6: 3: 2, v/v) |
|
|
1.5mL/min; 800rpm |
|
|
UV λ254 nm |
|
|
METHOD 2 |
|
|
Powder and extract roots and rhizomes of Glycyrrhiza inflata Bat. with 70% ethanol three times, each for 2 hours. Filter the extracts and evaporate under reduced pressure. |
|
|
Suspend the residue in distilled water and partition it with CH2Cl2, EtOAc, and n-butanol. After concentration under vacuum, analyse these fractions by DPPH-HPLC-MS screening method. As EtOAc extract of G. inflata is rich in radical scavengers, the EtOAc extract is further fractionated. |
|
|
Subject the EtOAc extract to silica gel column chromatography (2.0 kg, 100-200 mesh, 10 × 100 cm) with CH2Cl2-MeOH (60:1 - 1:1, v/v) as the mobile phase. Subject fractions 34-41, eluted with CH2Cl2-MeOH = 30: 1 (v/v), to silica gelcolumn chromatography (200 g, 200-300 mesh, 3.5 × 60 cm) with gradient using petroleum ether-EtOAc (10:1 - 1:1, v/v). Crystallize subfractions 75-95, eluted with CH2Cl2-MeOH (2:1, v/v), in methanol to give licochalcone A. |
|
|
METHOD 3 |
|
|
Extract the lyophilized and powdered roots of Glycyrrhiza uralensis with ethyl acetate three times at room temperature. Combine andf concentrate the extract in vacuo to give a residue. |
|
|
Dissolve the residue in ethyl acetate and partition it with n-hexane to give an active ethyl acetate residue by using the different solubilities. Subject the active residue to silica gel column (5.0 × 45.0 cm) and elute the column with ethyl acetate/n-hexane (1: 2, by vol) to give eight fractions (A-H). |
|
|
Subject fraction H to prep RP-18 column, elute it with methanol/water (1.5: 1, by vol) to give licochalcone A by the detection UV at 285 nm and flow rate at 5 mL/min. |
|
|
| Reference |
|
[1]
|
Fu, Y., et al. (2013). "Antioxidant and anti-inflammatory activities of six flavonoids separated from licorice." Food Chemistry 141(2): 1063-1071. |
|
[2]
|
Furuhashi, I., et al. (2005). "Inhibition by licochalcone A, a novel flavonoid isolated from liquorice root, of IL-1beta-induced PGE2 production in human skin fibroblasts." J Pharm Pharmacol 57(12): 1661-1666. |
|
[3]
|
Chu, X., et al. (2013). "Attenuation of allergic airway inflammation in a murine model of asthma by Licochalcone A." Immunopharmacol Immunotoxicol. |
|
[4]
|
Chu, X., et al. (2012). "Licochalcone a inhibits lipopolysaccharide-induced inflammatory response in vitro and in vivo." J Agric Food Chem 60(15): 3947-3954. |
|
[5]
|
Rafi, M. M., et al. (2000). "Modulation of bcl-2 and cytotoxicity by licochalcone-A, a novel estrogenic flavonoid." Anticancer Res 20(4): 2653-2658. |
|
[6]
|
Yuan, X., et al. (2013). "Licochalcone a-induced human bladder cancer t24 cells apoptosis triggered by mitochondria dysfunction and endoplasmic reticulum stress." Biomed Res Int 2013: 474272. |
|
[7]
|
Kim, Y. J., et al. (2013). "Licochalcone A Enhances Geldanamycin-Induced Apoptosis through Reactive Oxygen Species-Mediated Caspase Activation." Pharmacology 92(1-2): 49-59. |
|
[8]
|
Xiao, X.-y., et al. (2011). "Licochalcone A inhibits growth of gastric cancer cells by arresting cell cycle progression and inducing apoptosis." Cancer Letters 302(1): 69-75. |
|
[9]
|
Fu, Y., et al. (2004). "Licochalcone-A, a novel flavonoid isolated from licorice root (Glycyrrhiza glabra), causes G2 and late-G1 arrests in androgen-independent PC-3 prostate cancer cells." Biochemical and Biophysical Research Communications 322(1): 263-270. |
|
[10]
|
Kim, Y. H., et al. (2010). "Antiangiogenic effect of licochalcone A." Biochem Pharmacol 80(8): 1152-1159. |
|
[11]
|
Li, Y.-J., et al. (2011). "Screening and characterization of natural antioxidants in four Glycyrrhiza species by liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry." Journal of Chromatography A 1218(45): 8181-8191. |
|
[12]
|
Hao, H., et al. (2013). "Effect of Licochalcone A on Growth and Properties of Streptococcus suis." PLoS One 8(7): e67728. |
|
[13]
|
Kim, S. N., et al. (2008). "Licochalcone A inhibits the formation and bone resorptive activity of osteoclasts." Cell Biology International 32(9): 1064-1072. |
|
[14]
|
Won, S.-R., et al. (2007). "Licochalcone A: A lipase inhibitor from the roots of Glycyrrhiza uralensis." Food Research International 40(8): 1046-1050. |
|
[15]
|
Cui, S., et al. (2007). "Identification of Radix Glycyrrhizae by microemulsion thin-layer chromatography." Zhongcaoyao 38(4): 540-542. |
|
[16]
|
Zhang, J., et al. (2012). "Flavonoids content in Licorice residue determined by HPLC." Zhongguo Zhongyiyao Keji 19(3): 233-234. |
|
[17]
|
Wang, Q.-E., et al. (2004). "Isolation and purification of inflacoumarin A and licochalcone A from licorice by high-speed counter-current chromatography." Journal of Chromatography A 1048(1): 51-57. |
|
| Link to |
 Chinese Medicinal Material Images Database Chinese Medicinal Material Images Database
 Medicinal Plant Images Database Medicinal Plant Images Database
 Chinese Medicine Specimen Database Chinese Medicine Specimen Database
|
 Chinese Medicinal Material Images Database
Chinese Medicinal Material Images Database
 Medicinal Plant Images Database
Medicinal Plant Images Database
 Chinese Medicine Specimen Database
Chinese Medicine Specimen Database


